Atopic Dermatitis and Inflammation on the Depressions of the Thoracic Vertebrate (T1-T4) - A Proposal Based on Electroacupuncture-cupping Treatment
Willow JH Liu*
TCM Health Education & Research Center, Diamond Bar, USA
Received Date: 06/02/2025; Published Date: 10/03/2025
*Corresponding author: Willow JH Liu, TCM Health Education & Research Center. 888 Brea Canyon Rd, Suite 380, Diamond Bar, CA 91789, USA
Abstract
Background: Atopic dermatitis (AD) is a chronic, relapsing inflammatory disease that causes skin redness and pruritus and challenging to cure. However, our understanding of its pathology remains limited. Acupuncture and Chinese herbs have historically been used to treat AD, yet their mechanisms of action are not fully understood.
Objective: Elucidate the mechanisms underlying Willow’s AD quadruple combo therapy and explore the possible link between inflammation in the depressions below the 1st to 4th thoracic vertebrae (T1- T4 points) and the function of the thymus.
Methods: A patient with severe AD was treated with Willow’s AD quadruple combo therapy, an integrated regimen combing electroacupuncture (EA), cupping, herbal formula, and dietary modifications. Pricking cupping was performed on the tender points between T1 and T4 after EA.
Results: Before treatment, the patient confirmed tender points on the T1-T4 points. After treatment, the tenderness gradually diminished, along with skin pruritus and redness. All symptoms completely disappeared after one and a half months of the combined treatment and with no recurrence.
Conclusion: Willow’s AD quadruple combo therapy demonstrated significant efficacy of traditional Chinese medicine in alleviating symptoms of severe AD. The mechanism underlying this treatment is discussed.
Proposal: AD flair-ups may be linked to inflammation on T1-T4 points, where the spinal nerves extend and connect with the sympathetic nerves, the latter regulating various organs, including the thymus, which plays a key role in the immune system. EA treatment applied on T1-T4 points and both sides of these points may exert its effects by potentially regulating thymus function through the stimulation of sympathetic nerves. Research methods to test this proposal are outlined.
Keywords: Atopic Dermatitis (AD); Electroacupuncture (EA); Pricking cupping; Chinese Herbal Medicine (CMH); Traditional Chinese Medicine (TCM); Willow’s AD quadruple combo therapy
Introduction
Atopic dermatitis (AD), commonly known as eczema, is a prevalent inflammatory skin condition that affects both children and adults. The condition is influenced by various factors, including genetics, T helper 2 (Th2) immunity, disrupted gut and skin barrier microbiome, and food or environmental triggers [1-3]. Type 2 inflammation plays a pivotal role in AD by compromising the skin barrier, which is crucial for preventing water loss and blocking toxins from entering the body [4]. Despite these insights, the exact pathogenesis of AD remains incompletely understood.
Conventional treatment for AD typically includes persistent use of topical creams, gels, or ointment, and oral or injectable prednisone, immunosuppressants and biologics for individuals with moderate to severe disease who do not respond well to other treatment [5]. However, prednisone is not suitable for long-term use due to potential serious effects, and biologics are expensive.
As common methods in traditional Chinese medicine (TCM), Chinese herbal medicine (CHM) [6,7], acupuncture [8,9], and prickling cupping [10] have a long history of use in treating AD, and their efficacy and safety have been documented. The herbal ingredients [11,12] and acupoints [13,14] for AD have been reviewed. However, treatment outcomes vary between practitioners due to differences in herbal formulation or acupoints selection. Additionally, the mechanisms underlying these therapies need to be explored more thoroughly. Moreover, the mechanisms driving these therapies still need to be explored in greater detail.
This article presents a case of severe AD treated with Willow’s AD quadruple combo therapy, which including internal and external CHM, electroacupuncture (EA), cupping (including pricking cupping), and dietary modification, as well as proposed mechanism behind these treatments.
Case Presentation
A 74-year-old male with AD first visited the clinic on September 3, 2020.
Chief complaint: Intensive itching on the skin of the hands, arms, and lower legs. The eczema began on one instep three months prior. Conncerned about the side effects, the patient refused to take prednisone prescribed by a dermatologist and used Seaka cream, but it was ineffective. He was referred to a senior TCM practitioner and received treatment with CHM. However, his symptoms progressively worsened. He experienced swollen, heaviness and tightness in his legs, which impaired his ability to walk and forced him to remain in bed. Additionally, he frequently woke up during sleeping due to pinprick-like pain in his legs. In the week before his visit, foamy urine was observed.
History: The patient has a history of allergic rhinitis and hypertension. Since 2017, he had been taking doxazosin mesylate, carvedilol, and nifedipine for hypertension management.
In September 2016, he was hospitalized in Macao for one month due to kidney failure caused by prostate hypertrophy obstructing the urinary system. He underwent prostate surgery and began taking medication to protect his kidney function, which has since normalized.
The patient’s first onset of AD occurred in April 2016 and completely subsided after approximately one month of treatment with Willow’s quadruple combo therapy by the author.
Examination: The patient’s skin on the hands, forearms, and legs below the knees exhibited redness or darkening, swelling, and in some area, oozing, cracking, or crusting (Figure 1). During the examination, when the thumb was used to apply pressure to the depressions below the thoracic vertebrae T1, T2, T3, and T4 (T1-T4 points), the patient experienced pain.
Treatment: The patient underwent Willow’s AD quadruple combo therapy for 6 weeks. The details are outlined below.
- Electronic acupuncture (EA):
G32 x 1.5” acupuncture needles with 2 Hz electronic current applied for 20 minutes on the 12 back points first and then 14 front limb points. The acupoints were disinfected with 70% alchohol before needing.
12 back points: T1-T4 points and the eight side points (one inch on either side of the spinal line, parallel to T1-T4 points). T1-T4 points were punctured perpendicularly, while the eight side points were punctured at a 45-degrees angle towards the spinal line.
14 limb points: ST36, SP10, SP9, SP6, KI3, LI11, LI4 on both sides of the legs or arms. No electrical stimulation on ST-36. Needles were inserted perpendicularly into the skin.
Sessions were conducted twice per week in the first month and once per week after then.
- Cupping: After EA treatment on the back, plastic vacuum cupping was immediately applied above the T1-T4 points for about 5 minutes.
Pricking-cupping: Immediately after cupping, the painful point between T1 and T4 was punctured rapidly and repeatedly with a 7-star plum blossom needle (15 punctures). Cupping was then applied above the punctured area immediately afterward. The blood was cleaned, and cupping was repeated for three times. Then the punctured area was disinfected with iodine and covered with a pad to prevent infection. The procedure was performed on one tender point in each EA session, starting from the most painful point.
In addition to the tender T1-T4 points, this procedure was also performed on areas with oozing (a-shi points).
Note: Transparent small liquid beads were noticed above some of the needle holes on the 12 back points after cupping following the first EA treatment on the back, but no more after two sessions of EA treatment. Before the pricking-cupping, the practitioner should make sure that the patients was not taking blood thinner.
- CHM formula
Oral CHM formula: Willow’s AD formulated herbal extract, a mixture of herbal water extracts consists of Arnebiae Radix (zi cao) and other 8 CHMs, was prescribed to the patients. 10 grams of the mixed extract (equals to 50 grams raw materials) was dissolved in 30-40 ml of boiling water. Take twice per day.
External CHM formula: Willow’s AD formulated herbal powder, a mixture of 15 grams of ground CHM powder in a tea bag, which consists of Sophorae Flavescentis Radix (ku shen) and other 3 herbs, was prescribed. The tea bag was decocted with 300 ml of water in a pot, simmered for 20 minutes after boiling. Repeatedly wash the lesion area with the warm decoction twice per day. One tea bag was used for 2 days.
- Diet control: The patient was advised to avoid eating spicy and fried food, shelled seafood, and BBQ, as well as to refrain from drinking alcohol during treatment and for two months afterward. For dinner, a porridge made of a mixture of coix seeds and green bean (in a 4:1 ratio) was recommended.
Results
After 10 days of Willow’s AD quadruple combo therapy (three sessions of EA treatments), the patient reported a significant reduction in itchiness. The lesions gradually healed (Figure 2). After one and a half months of treatment, the patients had full recovered. In January 2021, three months after the TCM treatment, when the patient returned to Macao and visited his nephrologist, he was informed that his AD was likely caused by carvedilol as a side effect, prompting the discontinuation of the medication. A follow-up blood test showed all indices were normal, indicating that the CHM formula was safe and without side effects.
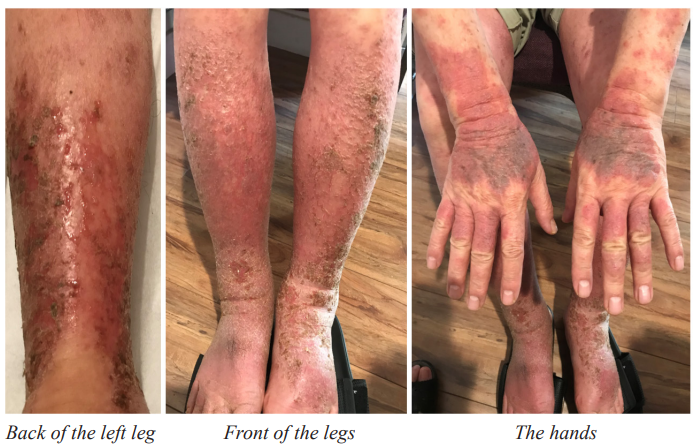
Figure 1: Pictures of the AD patient taken on 09/03/2020 before treatment.
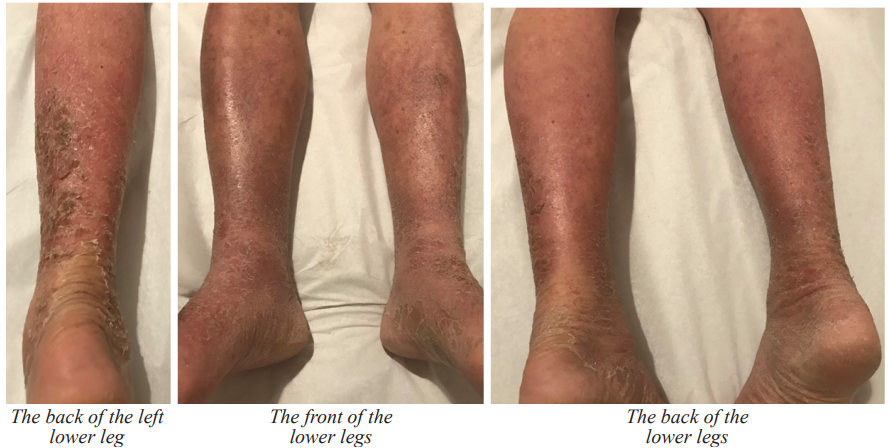
Figure 2: Pictures of the AD patients taken on 09/09/2020 (after 6 days of Willow’s AD quadruple combo therapy including 2 sessions of EA-Cupping treatment) and 09/16/2020 (after 13 days of Willow’s AD quadruple combo therapy including 3 sessions of EA-Cupping treatment).
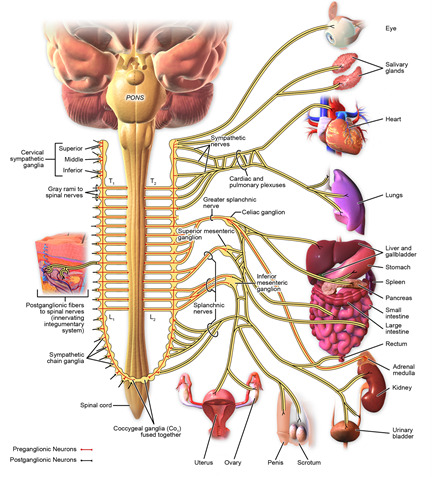
Figure 3: Sympathetic innervation.
Discussion
AD is a chronic and recurrent skin disease characterized by Th2 cell-mediated type 2 inflammation involving a multifactorial and complex interaction among epidermal barrier dysfunction, environmental factors, and immune dysregulation [15]. Recent research highlights epidermal lipid profiles, neuroimmune interactions, and microbial dysbiosis as emerging focal points in the pathophysiology of AD [2], but its etiology remains incompletely understood.
The thymus represents the primary site for T cell lymphopoiesis [16]. An increase in thymus size has been reported in children with active AD [17,18]. However, the involvement of thymus function in AD among adults requires further investigation.
TCM treatment for AD mainly encompasses internal and external herbal medicine, dietary therapy, acupuncture, and pricking cupping. Here, the author discusses the potential mechanisms underlying various TCM treatment approaches and proposes an association between inflammation at the T1-T4 points, the possible involvement of the sympathetic nervous system, and thymus in AD patients.
Thymus to AD
Since cytokines of type 2 immunity consistently predominate in the acute phase of AD, recent successes in systemic therapeutics for AD have focused on targeting cytokines produced by T cells [19]. The thymus is a crucial organ for immunity. Responsible for the creation of immune competence, represented by a multifunctional population of T lymphocytes, the thymus is able to control immune functions not only by supplementation of immune system with T cells but also by regulation of immunity indirectly by neurohormonal mechanisms. The thymus occupies a central position in the neuro-endocrine-immune network integrating its homeostatic tasks in the range of metabolic, procreative, regenerative, tolerogenic and defensive functions of the organism [20].
Previously, it was believed that thymic activity is primarily active during childhood [21]. However, recent studies revealed that the thymic lymphopoietic and endocrine functions are maintained in the adult and elderly individuals [20], albeit with varying intensities that are adjusted to different stages of life.
Clinic studies have shown the increased size of thymus among children with active AD compared with healthy controls. The larger size of thymus is compatible with increased thymic activity and emission of Tlymphocytes [17,18]. Thymectomy has been linked to a higher risk of autoimmune disease, developing cancer, and dying in adults [22]. But the link between the thymus and AD in adult have not been investigated.
Neuroimmunology to AD
The close communication between the immune and nervous systems in coordination with various skin cell populations orchestrates neuroimmune circuits that are critically involved in regulating pruritus in AD. Dysregulation of these neuroimmune circuits plays a key role in the pathophysiology of AD, causing inflammation, pruritus, pain, and barrier dysfunction [23].
Genetic and allergy trigger factors contribute to AD, activating both the nervous and the immune systems. Endogenous or exogenous trigger factors of AD such as microbes, irritants, or allergens can both directly or indirectly, via epidermal and immune cells, activate high-affinity receptors on peripheral sensory nerve endings. Activation of sensory nerve endings leads to depolarization and electrical transduction, which in turn leads to the release of further neuromodulators from central primary afferent nerve endings into the dorsal horn of spinal cord. Cytokines, chemokines, proteases, lipids, opioids, and ions excite and sensitize sensory nerve endings, which not only induces pruritus but further aggravates/perpetuates inflammation and skin barrier disruption [23].
Sympathetic and parasympathetic nerve fibers enter the thymus along with blood vessels and branch. The strict association between nerve fibers and blood vessels strongly supports the hypothesis that autonomous innervation is also involved in controlling T-cell trafficking, i.e., both entry of T cells into the thymus and their exit from the thymus as mature T cells [24,25].
Sympathetic nerves originate from the T1 and L3 segments of the spinal cord, first travelling to the sympathetic chain, and then to the organs and tissues controlled by these nerves (Figure 3). Therefore, the sympathetic nerves play a critical role in regulating the function of internal organs.
An early study reviewed that the input to the thymus, which originates from the superior cervical ganglion and extends to about the T3 level [26]. Later, it was reported that the thymus is innervated by nerve fibers originating from postganglionic cell bodies located in the superior cervical and stellate ganglia [25]. The superior cervical ganglion receives preganglionic fibers from the T1 spinal nerve, meaning that the T1 nerve is the main pathway for sympathetic innervation reaching the superior cervical ganglion [27]. Meanwhile, the stellate ganglion is formed by the fusion of the inferior cervical ganglion and the first thoracic (T1) ganglion, meaning that the T1 nerve directly contributes to the formation of the stellate ganglion, essentially becoming part of it when they merge [28].
These findings help explain the tender points between T1-T4 among the patients with moderate or severe AD, as well as the efficacy of the EA therapy on the 12 back points.
Food, microbiome, and AD
A crosstalk between commensals and the immune system has been well known. Alterations in this crosstalk are believed to affect the maturation of innate and adaptive immunity during early life [29]. Recent advances in understanding AD pathophysiology have revealed that skin and gut microbiome dysbiosis plays an important role in the disease [30-33].
Diet plays a significant role in shaping the microbiome, with experiments showing that dietary alterations can induce large, temporary microbial shifts within 24 h. Most functions of the microbiome are exerted through microbiome-derived metabolites. Gut flora and their metabolites actively participate in both B cell and T cell proliferation and differentiation, thus inducing protective antibody responses [33].
Type 2 immune responses are primarily triggered by the recognition of certain types of antigens, including food and airborne allergies and some bacteria and viruses, typically occur in peripheral tissues [34,35]. Foods that trigger AD include milk, eggs, junk foods, dairy, flower products gluten, nightshades, crab, shrimp, mutton, beef, and fish et al [35,36].
The elevated level of total serum IgE (sIgE) is one major hallmark of AD. Food allergy-specific IgE is produced during sensitization. B cells play several roles in the development of food allergy versus. Th2 cells regulate B cell class switching to IgE through their production of Interleukin-4 (IL-4) [37]. Recent animal study revealed that plasma cells in the thymus can secrete homeostatic sIgE, which can increase the number of mast cells locate at the mucosal surfaces of the gut and skin that correlating with the severity of anaphylaxis [38]. This discovery connected the thymus with allergies and anaphylaxis closely.
Coix (Semen Coicis) is used as both a food and a CHM in China. Pharmacological studies demonstrated it could regulate the intestinal microbiota and has anti-tumor and anti-inflammatory effects [39]. Therefore, coix was recommended to AD patients by the author.
CHM for AD
Pharmacological and clinical studies have confirmed that most CHMs used for AD treatment have anti-inflammatory, anti-allergic, antioxidant, and/or anti- angiogenic effects, which can attenuate AD through various mechanisms such as restoring the skin barrier while balancing Th1/Th2 cell levels and regulating the expression of cytokines and chemokines through a variety of mechanisms with few sides effect [12].
Some CHMs were demonstrated to have preliminary immunomodulatory effects on T cells and basophils. The active compounds in these CHMs have been found to have potent inhibition of immunoglobulin IgE, mast cell activation, and proinflammatory cytokine or signaling pathway, and have value for both IgE and none IgE- mediated food allergy [40].
In a statistic study using a national database in Taiwan, Forsythiae Fructus (lian qiao) was reported the most popular CHM used for treating eczema, followed by Coicis Semen (yi yi ren) and Taraxaci Herba (pu gong ying). While Xiao Feng San (XFS) was the most common used herbal formula [11]. The effect of gromwell (zi cao, Radix Lithospermi ) extract on AD was confirmed by both clinical and animal studies [41,42].
In addition to ingestion, CHMs are also topically used for AD treatment [43,44]. Phellodendri Chinensis Cortex (huang bai), Sophorae Flavescentis radix (ku shen), Cnidii Fructus (she chuang zi), and Dictamni Cortex (bai xian pi), which are most commonly used herbs for AD, were listed as the core CHMs having effects on the pathways on immune and metabolism systems different from Western medicine [40]. Besides, sulfur is also used alone or in combination with other CHMs for dermatological conditions including seborrheic AD [45].
It should be noted that, due to varying backgrounds and expertise, different TCM practitioners use different CHMs and acupuncture formulas for AD treatment, either based on classical formula or their own creations. Therefore, the treatment results can vary, and in some cases, may even worsen the symptoms. The senior TCM practitioner in this case serves an example.
Acupuncture for AD
Efficacy and safety of acupuncture treatment for eczema has been well-documented and reviewed [8,9]. The most commonly used acupoints for AD are ST36, SP6, SP10, LI4, LI11, and a-shi points (the points located on the lesion area) [13,14,46]. Other commonly used acupoints are sea points, confluence points, and back-shu points [13,46].
The immunomodulatory and anti-inflammatory mechanisms of acupuncture have been widely studied, particularly in how acupoints communicate with target organs via neuro-immune regulation has been widely studies. The multisystem anti-inflammatory effect of acupuncture involves the regulation of innate immune cells as well as adaptive immune cells, including macrophages, granulocytes, mast cells, and T cells [47,48].
The somatic afferents present in acupuncture-activated acupoints convey sensory signals to the spinal cord, brainstem, and hypothalamic neurons. Upon information integration in the brain, acupuncture further stimulates multiple neuro-immune pathways, including the cholinergic anti-inflammatory, vagus-adrenal medulla-dopamine, and sympathetic pathways, as well as the hypothalamus-pituitary-adrenal axis, ultimately acting immune cells via the release of crucial neurotransmitters and hormones. ST36, SP6, LI4, LI11, and KI3 were listed for their anti-inflammation effect and their mechanisms of the treatment was revealed [48].
Cupping and pricking-cupping for AD
Cupping therapy is an ancient healing technique performed by applying cups to selected skin areas and creating a negative pressure, either by heat or by suction [49]. Pricking-cupping is TCM therapy that involves puncturing the skin with sterile needles and then applying a cup to the area to draw small amount of blood through the needle holes. It differs from “bloodletting”, which is no longer used in the West [50].
Both cupping and pricking-cupping have been used to treat various skin diseases. The procedures, theories, and mechanism of cupping and pricking-cupping in dermatology have been detailed reviewed [51,52,10]. The proposed mechanisms of cupping include effects of subatmospheric pressure suction, promoting peripheral blood circulation, and improving immunity. Reported benefits of cupping therapy for AD include enhanced skin blood flow, changing of the skin’s biomechanical properties, increasing pain thresholds, improving local anaerobic metabolism, reducing inflammation, and modulation of the cellular immune system [52].
Pricking-cupping is particularly effective for relieving itching through three main mechanisms:
(1) The theory of "neuro-endocrine-immune network". By stimulating the acupoints and the nerve endings around the skin lesions to stimulate and regulate the endocrine system, the body releases certain hormones and neuropeptides, and acts on the immune system, so that immune cells derive protective polypeptide factors, thereby achieving the effect of relieving itching.
(2) Pricking-cupping can directly discharge endotoxins, toxic substances, excessive histamine, endothelin and other inflammatory mediators in the blood by releasing an appropriate amount of blood, thereby improving vascular function, reducing inflammatory reactions, and reducing patients' itching.
(3) In the early stage of inflammation, vascular congestion is a key factor in causing itching, and pricking cupping can directly improve the congestion of blood vessels, by reducing the pressure in the local small blood vessels, restoring the osmotic pressure of the blood vessel wall, prompting the extravasated fluid to return to normal circulation, promoting blood metabolism, and helping to relieve itching and inflammation [10].
Fire needle on lesioned skin combining with pricking-cupping has been report effective treating AD. Acupoints GV14, BL13, BL17, and BL20 were selected for pricking cupping [53].
Hypothesis of the link between inflammation on T1-T4 points to AD and asthma
Willow’s AD quadruple combo therapy has been successfully used to treat several dozen adult patients with moderate to severe AD. Pricking-cupping and external CHMs were applied only to severe AD patients with skin cracking, oozing, or crusting. In addition to the T1-T4 tender points, pricking-cupping was also applied to a-shi points (the lesion areas).
In TCM, the spinal line is part of the Governing Vessel (GV, Du Mai). Some acupoints in the GV lie in the depressions below the spinous processes of the thoracic vertebra. For example, GV 12 is located below T3, GV 13 is below T1. These points are commonly used to treat respiratory disorders like cough and asthma [54]. The author selected the T1-T4 points primarily based on the tenderness found during the patients’ initial examination.
Among the adult patients with moderate or severe AD treated by the author, only three patients with severe AD showed no obvious tenderness on the T1-T4 points during the initial examination. The number of tender points and the severity of tenderness were correlated with the severity of the symptoms. Patients who experience tenderness at the T1-T4 points reported a gradual reduction in tenderness, which eventually disappeared as their symptoms improved following treatment.
During the first session, transparent small liquid beads were commonly observed above some of the needle holes after cupping on the 12 back points. The author attributes the liquid to inflammatory exudate, as no more liquid beads was obbserved once tenderness subsided following several sessions of EA-cupping and pricking-cupping treatment.
The author observed this phenomenon in patients with asthma. The same 12 back acupoints and 10 points on the legs for EA therapy were also used for asthma patients. But the acupoints on the arms (LI-11 and LI-4 used for AD) were replaced with LU5 and LU9 (commonly used for asthma treatment).
Based on these observations and the understanding of neuroimmune interactions in allergic diseases, the author proposed that inflammation on the T1-T4 points may be linked to flair-ups of AD and asthma, both of which involve type 2 immune response.
Inflammation at these points can alter the regulatory function of the sympathetic nerves, subsequently impacting thymus function. The thymus is crucial for the development and maturation of T cells. EA treatment likely exerts its therapeutic effects by reduce inflammation and restore the regulatory function of the thymus through sympathetic nerve pathways.
The eight points on the sides of the spine, parallel to T1-T4, are located between Hua Tuo Jia Ji points (0.5 cun lateral to the lower border of each spinous process) and the first lateral line of the Foot-Taiyang Vessel (1.5 cun lateral to the lower border of each spinous process), which are traditionally used to treat respiratory and cardiovascular disorders [54]. The author chose the points 1 cun lateral to the spinal points and inserted the needles 45 degree toward the spine, based on the anatomical distance of the sympathetic truck from the midline of the spine.
The cardiac and pulmonary plexuses receive branches directly from the T1-T4 spinal cord segments (Figure 3). The pulmonary plexus innervates the larynx, trachea, bronchi and lung. In TCM, it is believed that “the lung is connected with skin and hair”. Both TCM theory and the anatomical discovery of the sympathetic nerve innervation provide insight into the mechanism of EA treatment using the 12 back points, for both AD and asthma patients – taking its effects by regulating the sympathetic nerves and reducing inflammation at these points.
Proposal for approach to test the hypothesis
To verify the author’s hypothesis of relationship between inflammation on T1-T4 points, sympathetic nerves, thymus function, and AD flair-up, the outline of the following clinical studies on Willow’s AD quadruple combo therapy to adult patient with moderate and severe AD patients are proposed.
- The study can be divided into 4 groups: blank + diet, CHM formula + diet, EA + cupping + diet, and Willow’s AD quadruple combo therapy (CHM + EA + cupping + diet)
- Tender points between T1-T4: exam the tender points between T1-T4 before and after treatment, and record the correlation of points numbers and severity of the tenderness with the symptoms of skin lesion and itchiness,
- Analysis of the blood released from pricking-cupping on T1-T4 points: collect the blood containing blood and inflammatory extrude using a tube containing heparin. Use microscope to observe the cells and use LC-MS to identify the components in the collected sample separately.
- Blood analysis: analyze serum IgE [38] and AD specific cytokines thymus and activation-regulated chemokine (TARC) [55] before and after the treatment.
- Gut microbiome analysis: compare the difference before and after treatment.
- Measure the size of the thymus: to evaluate the involvement of the thymus to AD onset, compare the size difference before and after treatment.
Funding sources: None
Conflicts of interest: None disclosed.
Patient consent: The patient provided written consent.
References
- Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, and Eichenfield LF. Atopic dermatitis: pathogenesis. Semin Cutan Med Surg, 2017; 36(3): 100-103. doi: 10.12788/j.sder.2017.036.
- Kim J, Kim BE, and Leung DYM. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc,2019; 40: 84–92. doi: 2500/aap.2019.40.4202
- Boothe WD, Tarbox JA, and Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol, 2017; 1027: 21-37. doi: 10.1007/978-3-319-64804-0_3.
- Beck LA, Cork MJ, Amagai M, BenedettoAD, Kabashima K, Hamilton JD, et al. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov, 2022; 2(5): 100131. doi: 10.1016/j.xjidi.2022.100131.
- Armario-Hita JC, Galán-Gutiérrez M, Dodero-Anillo JM, Dodero-AnilloJM, Carrascosa J M, and Ruiz-Villaverde R. Updated review on treatment of atopic dermatitis. J Investig Allergol Clin Immunol, 2023; 33(3): 158-167. doi: 10.18176/jiaci.0906.
- Cai C, Sun XY, Liu L, Zhou YQ, Hong S, Wang J, et al. Efficacy and safety of Chinese herbal medicine for atopic dermatitis: Evidence from eight high-quality randomized placebo-controlled trials. Front Pharmacol, 2022; 13: 927304. doi: 10.3389/fphar.2022.927304.
- Kwon CY, Lee B, Kim S, Lee J, Park M, Kim Effectiveness and safety of herbal medicine for atopic dermatitis: an overview of systematic reviews. Evid Based Complement Alternat Med, 2020: 4140692.
- Jiao RM, Yang ZY, Wang Y, Zhou J, Zeng YX, and Liu ZS. The effectiveness and safety of acupuncture for patients with atopic eczema: a systematic review and meta-analysis. Acupunct Med,. 2020; 38: 3-14. doi: 10.1177/0964528419871058.
- Li XH, Liang LJ, Li S, Wang CY, Cucco A, Du XH, et al. Effect of acupuncture in eczema: An overview of systematic reviews. Complement Ther Med, 2023; 73: 102925. doi: 10.1016/j.ctim.2023.102925.
- Guo Y, Pang H, and Luo JH. Research progress of pricking blood therapy for pruritus dermatosis. Traditional Chinese Medicine (Hans), 2024; 13(9): 2123-2128. Doi:12677/tcm.2024.139316.
- Chen HY,Lin YH, Hu S, Yang SH, Chen JL, and Chen YC. Identifying Chinese herbal medicine network for eczema: Implications from a nationwide prescription database. Evid Based Complement Alternat Med, 2015: 347164. doi: 1155/2015/347164.
- Yan FG, Li F, Liu JF, Ye SQ, Zhang Yu, Jia JJ, et al. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomed Pharmacother, 2020; 127: 110142. doi: 10.1016/j.biopha.2020.110142.
- Zeng ZW, Li M, Zeng YJ, Zhang JL, Zhao YJ, Lin YX, et al. Potential acupoint prescribed and outcome reporting for acupuncture in atopic eczema: a scoping review. Evidence based complementary and alternative medicine. Evid Based Complement Alternat Med, 2021: 9994824. doi: 10.1155/2021/9994824.
- Park JG, Lee H, Yeom M, Chae Y, Park HJ, Kim K. Effect of acupuncture treatment in patients with mild to moderate atopic dermatitis: a randomized, participant- and assessor-blind sham-controlled trial. BMC Complementary Medicine and Therapies, 2021; 21(1): 132. doi: 10.1186/s12906-021-03306-1.
- LloydCM and Snelgrove RJ. Type 2 immunity: Expanding our view. Sci Immunol, 2018; 3(25): eaat1604. doi: 10.1126/sciimmunol.aat1604.
- Thapa P and Farber D. The role of the thymus in the immune response. Thorac Surg Clin. 2019; 29(2):123-131. doi: 1016/j.thorsurg.2018.12.001.
- Olesen AB, Andersen G, Jeppesen DJ, Benn CS, Juul S, and Thestrup-Pedersen Thymus is enlarged in children with current atopic dermatitis. A cross-sectional study. Acta Derm Venereol, 2005; 85(3): 240-243. doi: 10.1080/00015550510026352.
- Hossny E, Sakr H, El-Owaidy R, and El-Mekkawy A. Thymus gland assessment in infants and children with atopic dermatitis. Egypt J Pediatr Allergy Immunol, 2023; 21(1): 9-17. doi:21608/ejpa.2023.294363.
- Alsabbagh M, Ismaeel A. The role of cytokines in atopic dermatitis: a breakthrough in immunopathogenesis and treatment. Acta Dermatovenerol Alp Pannonica Adriat, 2022; 31(1): 13-31. doi: 10.15570/actaapa.2022.3.
- Dabrowski MJ, Dabrowski MI, and Stankiewicz W. The thymus in neuro-endocrine-immune network. Cent Eur J Immunol, 2011; 36(3): 188-192.
- Rezzani R, Nardo L, Favero G, Peroni M, and Rodella LF. Thymus and aging: morphological, radiological, and functional overview. Age (Dordr), 2014; 36:313–351. doi: 10.1007/s11357-013-9564-5.
- Kooshesh KA, Foy BH, Sykes DB, Gustafsson K, Scadden Health consequences of thymus removal in adults. N Engl J Med, 2023; 389(5):406-417. doi: 10.1056/NEJMoa2302892
- Steinhoff M, Ahmad F, Pandey A, Datsi A, AlHammadi A, Al-Khawaga S. et al. Neuroimmune communication regulating pruritus in atopic dermatitis. J Allergy Clin Immunol, 2022; 149(6): 1875-1898. doi: 10.1016/j.jaci.2022.03.010
- Mignini F, Sabbatini M, Mattioli L, Cosenza M, Artico M, and Cavallotti C. Neuro-immune modulation of the thymus microenvironment (Review). Int J Mol Med, 2014; 33(6): 1392-1400. doi: 10.3892/ijmm.2014.1709
- Remien K and Jan A. Anatomy, head and neck, thymus. StatPearls Publishing, Treasure Island, Florida, 2023.
- Nance DM and Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun, 2007; 21(6): 736–745. doi: 10.1016/j.bbi.2007.03.008.
- Maningat AL and Munakomi S. Neuroanatomy, Superior Cervical Ganglion. StatPearls Publishing, Treasure Island, Florida, 2023.
- Mehrotra M, Reddy V, and Singh P. Neuroanatomy, Stellate Ganglion. StatPearls Publishing, Treasure Island, Florida, 2023.
- Zheng DP, Liwinski T, and Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res,2020; 30: 492-506. doi: 10.1038/s41422-020-0332-7.
- KimJE and Kim Microbiome of the skin and gut in atopic dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J Clin Med, 2019; 8(4): 444. doi: 10.3390/jcm8040444
- Raglin H. Gut microbiome has significant impact on atopic dermatitis. Dermatology Times, 2023; 44(10).
- Li W and Yosipovitch G. The role of the microbiome and microbiome-derived metabolites in atopic dermatitis and non-histaminergic itch. Am J Clin Dermatol, 2020; 21(Suppl 1): 44-50. doi: 10.1007/s40257-020-00538-8.
- Singh RK, Chang HW, Yan D, Lee KM, D, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med, 2017; 15(1): 73. doi: 10.1186/s12967-017-1175-y
- Nosrati A, Afifi L, Danesh MJ, Lee K, Yan D, Beroukhim K, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat, 2017; 28(6): 523–538. doi: 10.1080/09546634.2016.1278071.
- Chang A, Robison R, Cai M, and Singh AM. Natural history of food triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract, 2016; 4(2): 229–236. doi: 10.1016/j.jaip.2015.08.006.
- Hu YQ and Zhang JZ. Inhalation and food allergen-specific immunoglobulin E in the Serum of 156 patients with atopic dermatitis and patients' subjective causative factors. J Peking U. (Health Science), 2020; 52(5): 980-984.
- Udemgba C and Lin B cells and food allergy. Curr Opin Pediatr, 2021; 33(6): 625-632. doi: 10.1097/MOP.0000000000001050
- Kwon DI, Park ES, Kim M, Choi YH, Lee MS, Joo SH, et al. Homeostatic serum IgE is secreted by plasma cells in the thymus and enhances mast cell survival. Nat Commun, 2022; 13(1): 1418. doi: 10.1038/s41467-022-29032-x.
- Li HJ, Peng LX, Yin F, Fang JH, Cai LT, Zhang CJ, et al. Research on coix seed as a food and medicinal resource, it's chemical components and their pharmacological activities: A review. J Ethnopharmacol, 2024; 319(3): 117309. doi: 10.1016/j.jep.2023.117309.
- Wang ZX, Wang ZZ, and Geliebter J, Tiwari R, Li Traditional Chinese medicine for food allergy and eczema. Ann Allergy Asthma Immunol, 2021; 126: 639-654. doi: 10.1016/j.anai.2020.12.002
- Cho HR, Cho Y, Kim J, Seo DB, Kim SH, Lee SJ, et al. The effect of gromwell (Lithospermum erythrorhizon) extract on the stratum corneum hydration and ceramides content in atopic dermatitis patients. Ann Dermatol, 2008; 20(2): 56-66. doi: 5021/ad.2008.20.2.56
- Kim YR, Cho SY, Seo DB,Kim SH, Lee SJ, and Cho Effects of oral intake of gromwell water fraction on ceramides content and the development of atopic dermatitis in NC/Nga mice. Korean J Food Sci Technol, 2009; 41(5): 547-551.
- Wang MC, Chou YT, Kao MC, Lin QY, Chang SY, and Chen HY. Topical Chinese herbal medicine in treating atopic dermatitis (eczema): A systematic review and meta-analysis with core herbs exploration. J Ethnopharmacol, 2023; 317: 116790. doi: 10.1016/j.jep.2023.116790.
- Gu S, Yang AW, Li CG, Lu CJ, and Xue CC. Topical application of Chinese herbal medicine for atopic eczema: A systematic review with a meta-analysis. Dermatology, 2014; 228(4): 294–302. doi: 10.1159/000360526.
- Gupta AK and Nicol K. The use of sulfur in dermatology affiliations J Drugs Dermatol,2004; 3(4): 427-431.
- Lin HL and Lin LC. Study on acupoint selection rules in acupuncture treatment for chronic eczema using data mining technology. Shanghai J Acupunct Moxibustion, 2022; 41(1): 102-106.
- Li NC, Guo Y, Gong YN, Zhang Y, Fan W, Yao KF,et al. The anti-inflammatory actions and mechanisms of acupuncture from acupoint to target organs via neuro-immune regulation. J Inflamm Res, 2021; 14: 7191–7224. doi: 2147/JIR.S341581
- Wang M, Liu WL, Ge JY, and Liu SB. The immunomodulatory mechanisms for acupuncture practice. Front Immunol, 2023; 141147718. doi: 10.3389/fimmu.2023.1147718
- Al-Bedah AMN, Elsubai IS, Qureshi NA, Aboushanab TS, Ali GIM, El-Olemy AT, et al. The medical perspective of cupping therapy: Effects and mechanisms of action. J Tradit Complement Med, 2019; 9(2): 90–97. doi: 1016/j.jtcme.2018.03.003
- Greenstone G. The history of bloodletting. BCMJ, 2010; 52(10):12-14.
- Aboushanab TS and AlSanad S. Cupping therapy: An overview from a modern medicine perspective. J Acupunct Meridian Stud, 2018; 11(3): 83-87. doi: 10.1016/j.jams.2018.02.001.
- Soliman Y, Hamed N, and Khachemoune A. Cupping in dermatology: a critical review and update. Acta Dermatovenerol Alp Pannonica Adriat, 2018; 27:,103-107.
- Zhu J, Wu YF, Liu Y, and Wu YC. Efficacy observation of blood-letting puncturing and cupping combined with fire needle for chronic eczema due to blood deficiency and wind causing dryness and its effect on quality of life. Shanghai J Acu-mox, 2021; 40(4):,481-486. (in Chinese)
- Cheng XN. Chinese Acupuncture and Moxibustion. Foreign Language Press, Beijing, 1999.
- Himadri, Renu George R, Mathew L, Shanmugam V, Mani T, Jeyaseelan L. The role of thymus and activation-regulated chemokine as a marker of severity of atopic dermatitis. J Am Acad Dermatol,2021; 84(2): 545-547.

