Narrative Review of Bilateral Congenital Hydronephrosis in a Newborn: A Multidisciplinary Approach
Yesenia Brito*, Cristian J Contreras Flores, Ana I Gonzalez, Arielle N Washington and Mohamed N Jabri
Department of Pediatrics, Saint George’s University School of Medicine, Grenada
Received Date: 04/05/2024; Published Date: 17/09/2024
*Corresponding author: Yesenia Brito, Department of Pediatrics, Saint George’s University School of Medicine, Grenada
Abstract
Congenital hydronephrosis develops from an obstruction of urinary flow at the renal pelvis and ureter. There are three major presentations of congenital hydronephrosis: a blockage in the urinary tract, vesicoureteral reflux, and delay in the development of the ureters. Infants with congenital hydronephrosis are typically asymptomatic; however, in severe cases, lack of appetite, swollen abdomen, and frequent urinary tract infections may be seen. During the antenatal period, fetal ultrasound may detect hydronephrosis and postnatal ultrasound may be performed to locate the blockage or anatomical abnormalities within the urinary system.
The management of congenital hydronephrosis involves conservative treatment in most cases and requires continuous monitoring. Cases demonstrating severe renal dysfunction, severe hydronephrosis, bilateral renal involvement, or associated posterior urethral valves should be recommended for surgical intervention to prevent chronic kidney disease. The overall prognosis of congenital hydronephrosis depends on the duration and severity of obstruction.
We present a case report of a newborn diagnosed with bilateral hydronephrosis, highlighting key aspects of the patient's history, clinical course, diagnostic findings, and management.
Categories: Pediatrics; Radiology; Urology
Keywords: Bilateral hydronephrosis; Pelvocaliectasis; Urine flow obstruction; Vesicoureteral reflux; Congenital hydronephrosis
Introduction
Congenital hydronephrosis refers to the dilation of the renal pelvis and/or calyces due to an interruption in the natural flow of urine [1]. A series of anomalies can affect the urinary tract and lead to hydronephrosis; these include abdominal masses, vesicoureteral reflux, and blockages along the tract [1]. Hydronephrosis can often be detected during the antenatal period via ultrasound; these cases are usually transient since two-thirds resolve spontaneously [2]. Some challenges to the early detection of hydronephrosis include oligohydramnios and maternal obesity [2]. Once detected, follow-up frequency can vary depending on the severity of the condition and the presence of gestational complications. Bilateral fetal hydronephrosis, for example, should be monitored every four to six weeks [3]. A postnatal ultrasound is recommended during the first seven days of life to measure renal pelvis diameter and pinpoint renal abnormalities [4]. Ultrasounds revealing a renal pelvis diameter >10 mm or a Grade 3-4 hydronephrosis should be followed by voiding cystourethrogram to look for vesicoureteral reflux [4]. If a Grade 3-4 hydronephrosis is not associated with vesicoureteral reflux, a diuretic renogram can help clinicians pinpoint areas of obstruction in the renal system [3].
Treatment plans are based on the cause and severity of the hydronephrosis. Using ultrasound findings, the Society for Fetal Urology created a tier system that classifies hydronephrosis into four grades [5]. Grades 1-3 can be regularly followed up with ultrasound to assess improvements or changes since these are the most likely to resolve spontaneously [1]. Grade 4 hydronephrosis, on the other hand, might require surgical intervention if there is renal damage or a significant decrease in renal function upon regular reassessment of the patient [1]. Congenital hydronephrosis places patients at an elevated risk of urinary tract infections; therefore, antibiotic prophylaxis is recommended for those with Grades 3-4 or those with a diagnosed vesicoureteral reflux [3].
Physicians should not rely on the Society for Fetal Urology Grading System alone when managing a patient with hydronephrosis, as approaches can vary based on the anatomical structures affected and the extent of renal involvement. Obstructions due to posterior urethral valves will require cystoscopic ablation, while conservative management is recommended in those with obstructive patterns that maintain a Differential Renal Function (DRF) above 40% [3]. Cortical thinning in a Grade 4 hydronephrosis case will indicate pyeloplasty [5], while the presence of medullary thinning without renal damage could be followed with a regular ultrasound [1]. Increases in medullary thinning between ultrasounds can skew management toward pyeloplasty or surgical correction, especially if the medullary thickness falls under 3 millimeters [6]. When pyeloplasty is warranted, DRF and renal pelvic collagen ratio can be used to predict the probability of postoperative renal function improvement. Those with a DRF < 35% have been shown to have poor postoperative outcomes. They also tend to have a higher collagen percentage in the renal muscle layer [7]. Declining renal function is accompanied by increased collagen formation, which restricts the renal pelvis and can lead to irreversible and permanent damage to the renal structure [7].
Overall, the management of congenital hydronephrosis tends to adhere to a conservative approach, emphasizing follow-up imaging and regular renal function assessments. Surgery should be reserved for those with declining renal function, associated posterior urethral valves, severe hydronephrosis, or bilateral renal involvement [3].
Case Presentation
We present a newborn male with a birth weight of 2.75 kg (6 lb 1 oz) and a birth length of 19.5 inches (49.53 cm), which was delivered via cesarean section at a gestational age of 37 weeks and 2 days. It is essential to note that this represents the mother's third pregnancy, with a medical history significant for thrombosis during her previous pregnancies. As a prophylactic measure, she was prescribed enoxaparin. The mother reported experiencing pronounced leg swelling and increased pain during the current pregnancy, symptoms not observed in her prior pregnancies. She underwent a cesarean section at 37 weeks due to oligohydramnios during her last pregnancy. The decision for the cesarean section was also influenced by the diagnosis of congenital hydronephrosis in the fetus, as identified through antenatal ultrasound at 5 months gestation. The mother denied a history of hypertension and diabetes mellitus, illicit drugs, or alcohol use during pregnancy.
The Apgar scores at one and five minutes were 9. The newborn had no dysmorphic features and normal physical examinations, including head, eyes, ears, nose, throat, chest, abdomen, pulses, hips, normal male genitalia, extremities, and neurologic assessments. The infant demonstrated healthy feeding patterns throughout the hospitalization and exhibited no concerning issues.
A renal ultrasound was ordered to assess renal anatomy and the severity of the condition that had been previously detected during the antenatal ultrasound. The kidneys revealed mild right pelvocaliectasis and minimal dilation of the left renal pelvis (Figure 1).
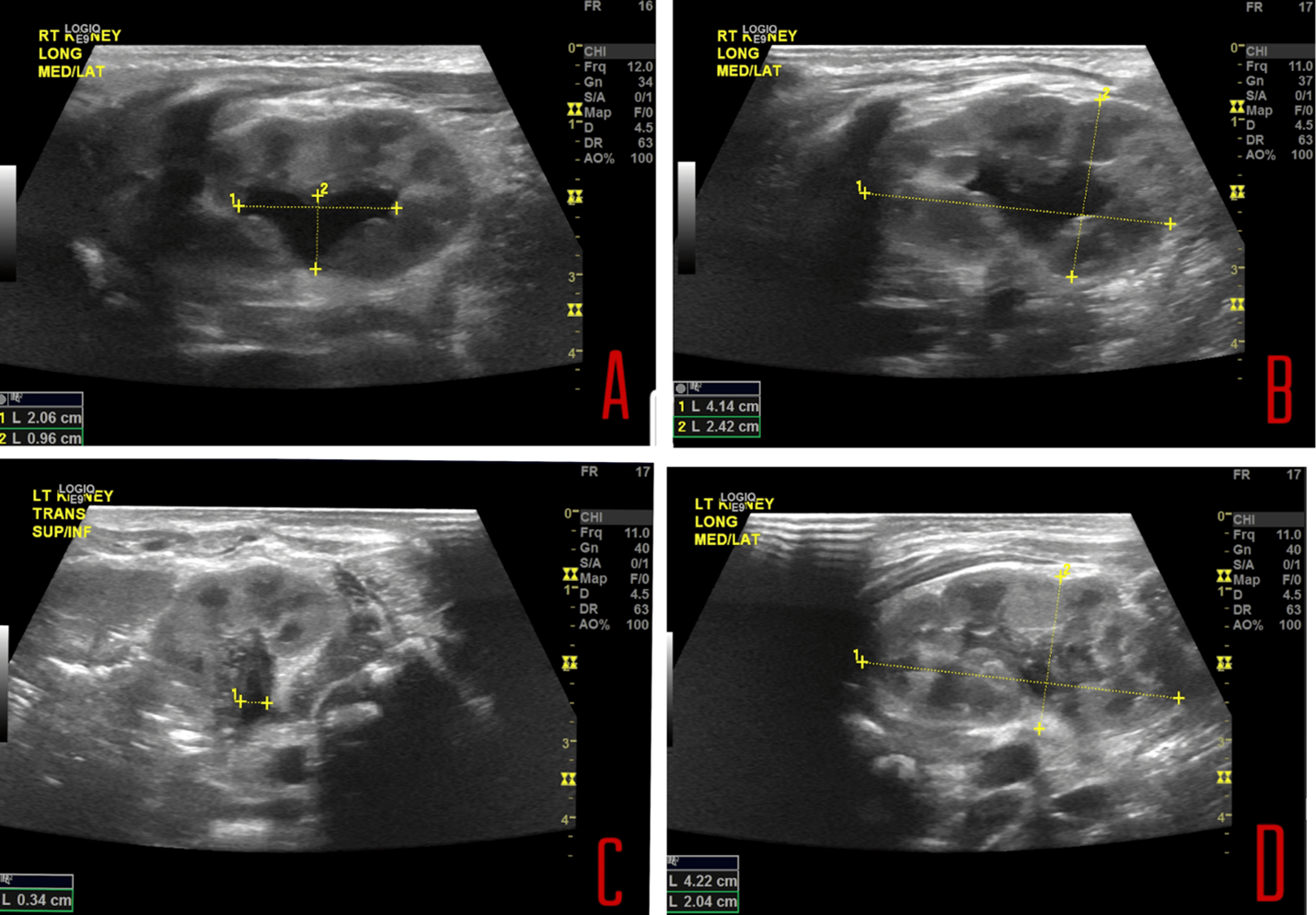
Figure 1: (A) Mild right-sided pelvocaliectasis and (B) The right kidney measures 4.1 cm in length. (C) Minimal dilatation in the left renal pelvis. (D) The left kidney measures 4.2 cm in length.
The infant was discharged at the age of 2 days, with a discharge weight of 2.63 kg (5 lb 12.7 oz) and a discharge length of 49.5 cm (19.5"). The patient was discharged in stable condition and referred to a urologist for further evaluation of congenital hydronephrosis. Primary care follow-up was scheduled for 3 days post-discharge.
A follow-up consultation with the urologist was conducted to discuss the results of the renal bladder ultrasound (RBUS) performed on April 15, which confirmed persistent mild bilateral pelviectasis with no other new abnormalities of the kidneys. The assessment was somewhat limited due to an under-distended bladder. Based on these findings, the urologist recommended continuing to monitor the patient with regular ultrasound examinations, with the next scheduled for August to assess any progression or resolution of the pelviectasis, as well as to evaluate the child's physical growth and development. The urologist also emphasized the importance of maintaining proper hydration to ensure normal urine flow and advised the mother to be vigilant for symptoms of a urinary tract infection (UTI), such as fever, irritability, or poor feeding, to address any potential complications promptly.
This case highlights the incidental discovery of congenital hydronephrosis in a newborn, emphasizing the need for a multidisciplinary approach involving neonatologists, pediatric urologists, and radiologists. The infant's overall health and positive outcomes from various screenings and examinations contribute to a favorable prognosis.
Discussion
In our case patient had isolated left sided PAPVC with right meandering pulmonary vein. Both of them are rare presentations of pulmonary venous anomaly.
Embryology of pulmonary veins is a complicated process. Blood returning from the lung buds initially drain into the splanchnic plexus which in turn eventually drains into paired cardinal and umbilic vitelline veins. Right superior vena cava is formed from the right cardinal system whereas left cardinal system mostly disappears and may potentially
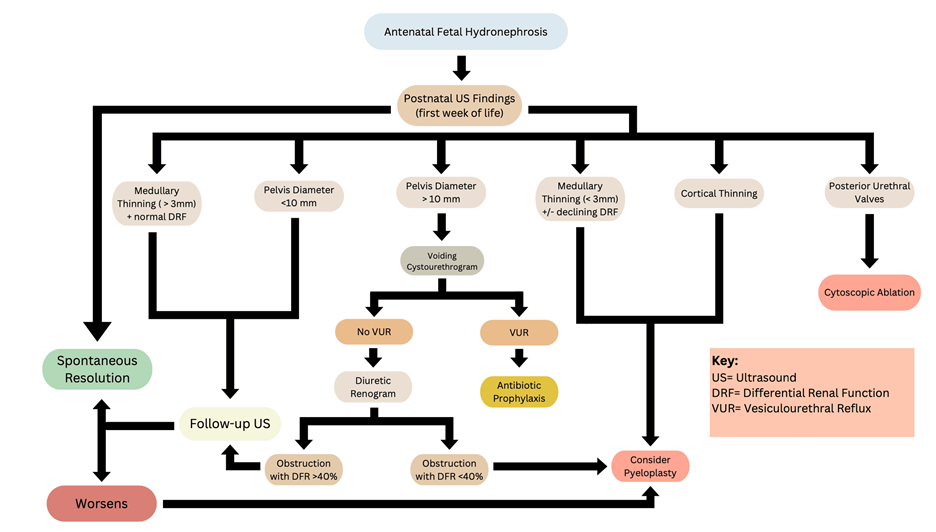
Figure 2: Management and expected outcomes of Congenital Hydronephrosis [1-7].
Image Credits: Yesenia Brito, Cristian J. Contreras Flores, Ana I. Gonzalez, Arielle N. Washington, Mohamed N. Jabri
While the initial renal ultrasound is deferred until the second day of neonatal life, the debate over using a cystourethrogram persists [10]. Nevertheless, it is recommended for all patients with antenatal hydronephrosis. In cases of suspected bladder outlet obstruction, a voiding cystourethrogram is performed within the first few days of life, and to rule out vesicoureteral reflux, it is carried out within the first month of life [10]. A radionuclide renal scan serves as a valuable guide for intervention by highlighting the differential function and drainage of the hydronephrotic fetal kidney. Implementing the Society for Fetal Urology's grading system for hydronephrosis is essential for classifying the degree of nephrosis of the fetal kidney. Despite this, prenatal hydronephrosis may often resolve spontaneously with the maturation of tubular function and an increasing ability for reabsorption [10]. In cases necessitating surgical intervention for severe hydronephrosis, treatment options include pyeloplasty for pelvic-ureteric junction obstruction when renal function is below 40%, nephrectomy for dysplastic non-functional kidneys, endoscopic valve ablation for posterior urethral valves, and ureteric reimplantation for vesicoureteric reflux [12]. This comprehensive approach underscores the nuanced management required in pediatric urology, balancing conservative strategies with targeted interventions based on the severity and nature of the condition.
While children with mild antenatal renal pelvic dilatation can often be discharged early, those with moderate to severe hydronephrosis necessitate vigilant monitoring by a multidisciplinary team. A study by Wolleneberg and colleagues [13] revealed that two-thirds of patients with severe antenatal renal pelvic dilatation required postnatal treatment, underscoring the importance of close follow-up in a specialized pediatric center. This approach involves a collaborative effort among various specialists to ensure comprehensive care for these children [13]. As the natural history and course of prenatally detected hydronephrosis are still being clinically defined, it becomes evident that collaborative efforts are indispensable. Through these collaborative efforts, proper clinical support can be provided, shedding light on the nuanced aspects of prenatally diagnosed hydronephrosis [14]. This multidisciplinary approach ensures thorough care and advances our understanding of the condition's complexities.
Over the past decade, prenatally detected hydronephrosis treatment management has significantly transformed. Previously, early surgical intervention upon diagnosis was the preferred approach, but there has been a paradigm shift towards a preference for close observation until indications of renal compromise become apparent. This updated strategy is grounded in the understanding that mild hydronephrosis commonly resolves spontaneously, restoring renal function to normal levels. A study analyzed by Koff, Jung Lim, and colleagues reported an encouraging 85% resolution of renal pelvic dilatation without surgical intervention in patients with prenatal hydronephrosis [15].
In essence, mild fetal hydronephrosis is often benign, and surgical intervention is usually unnecessary. However, the potential for poor outcomes increases for moderate to severe cases, necessitating surgical intervention. Consequently, managing fetal hydronephrosis requires a meticulous diagnostic protocol, emphasizing the critical importance of close antenatal and postnatal follow-up. For our case, surgical management is not expected to become necessary. However, it is important to continue monitoring renal anatomy and function until there is a complete resolution of the condition. Surgical intervention becomes a consideration if signs of renal compromise manifest [14]. This nuanced approach acknowledges the spectrum of severity in fetal hydronephrosis and underscores the need for personalized care based on the evolving clinical picture.
The case report on bilateral congenital hydronephrosis in a newborn male provides significant insights relevant to current practices in pediatric urology. Highlighting a case of prenatal detection and postnatal confirmation and management, the study underscores the evolving approach towards conservative management in the field. This reflects a broader trend in pediatric urology where the reliance on antenatal ultrasounds has facilitated early detection and tailored interventions, aiming to optimize renal function preservation while minimizing invasive procedures. The study's emphasis on regular monitoring and nuanced treatment decisions based on ultrasound findings and progression aligns with current recommendations advocating personalized care strategies. These findings contribute to the ongoing discourse on the best practices for managing congenital hydronephrosis, stressing the importance of a multidisciplinary approach involving neonatologists, pediatric nephrologists, and radiologists to ensure comprehensive care. This case report enhances our understanding of congenital hydronephrosis management and reinforces the need for ongoing research to refine the criteria for intervention versus continued observation.
Conclusion
Congenital hydronephrosis, stemming from anatomic abnormalities or blockages, can pose a threat to renal function if not promptly addressed. The most concerning complication is the progression to chronic kidney disease and eventual end-stage renal disease. While congenital hydronephrosis is often asymptomatic in infants or discovered incidentally during routine fetal ultrasound, the standard protocol for workup and management involves continuous monitoring and conservative measures for mild cases. For moderate to severe instances, surgical intervention can become necessary. However, recent research advocates for a multidisciplinary approach, bringing together neonatologists, pediatric urologists, and radiologists. This collaborative team can offer comprehensive care for infants with congenital hydronephrosis, ensuring accurate and timely diagnosis. This case report presents a mild case of congenital hydronephrosis, which was initially detected and monitored during the antenatal period. Renal ultrasound was performed postnatally to assess the extent of the condition, and a follow-up with a urologist was recommended. Although this case is expected to be resolved with conservative management alone, it was deemed appropriate to continue monitoring the patient to detect complications at the early stages and evaluate the need for a different course of action for complete resolution.
References
- Kohno M, Ogawa T, Kojima Y, et al. Pediatric congenital hydronephrosis (ureteropelvic junction obstruction): Medical management guide. International Journal of Urology, 2020; 27(5): 369-376. doi: 1111/iju.14207.
- Has R, Sarac ST. Prenatal diagnosis and findings in ureteropelvic junction type hydronephrosis. Frontiers in pediatrics, 2020; 8: 492. doi: 3389/fped.2020.00492.
- Sinha A, Bagga A, Krishna A, et al. Revised guidelines on the management of Antenatal Hydronephrosis. Indian journal of nephrology, 2013; 23(2): 83-97. doi: 4103/0971-4065.109403.
- Chiodini B, Ghassemi M, Khelif K, et al. Clinical outcome of children with antenatally diagnosed hydronephrosis. Frontiers in, 2019; 7: 103. doi: 3389/fped.2019.00103.
- Onen A. Grading of hydronephrosis: an ongoing challenge. Frontiers in Pediatrics, 2020; 8: 458. doi: 3389/fped.2020.00458.
- Kwiatkowski K, Lence T, Edwards AB, et al. The utility of renal medullary pyramidal thickness measurements on the first and second postnatal ultrasound in infants with congenital hydronephrosis. Journal of pediatric urology, 2023; 19(3): 309-e1. doi: 1016/j.jpurol.2023.01.002.
- Ulusoy O, Aydın E, Ateş O, et al. Clues for the early loss of renal function in congenital hydronephrosis: analysis of renal pelvis collagen ratio, diuresis renography, and upper urinary tract morphology. Journal of Pediatric Urology, 2023; 19 (2): 197.e1-197.e7. doi: 1016/j.jpurol.2022.11.018.
- Bingham G, Rentea RM. Posterior Urethral Valve. StatPearls Publishing, Treasure Island (FL); 2023.
- Berrocal T, Pinilla I, Gutiérrez J, et al. Mild hydronephrosis in newborns and infants: Can ultrasound predict the presence of vesicoureteral reflux. Pediatric Nephrology, 2007; 22(1): 91-96. doi: 1007/s00467-006-0285-1.
- Herndon CD. Antenatal hydronephrosis: Differential Diagnosis, evaluation, and treatment options. Scientific World Journal, 2006; 1(6): 2345-2365. doi: 1100/tsw.2006.366.
- Faiz S, Zaveri MP, Perry JC, et al. Role of antibiotic prophylaxis in the management of antenatal hydronephrosis, vesicoureteral reflux, and ureterocele in infants, 2020; 12(7): e9064. doi: 7759/cureus.9064.
- Chertin B, Pollack A, Koulikov D, et at. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: lessons learned after 16 years of follow-up. Eur Urol, 2006; 49: 734-738. doi: 1016/j.eururo.2006.01.046.
- Wollenberg A, Neuhaus TJ, Willi UV, et al. Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol, 2005; 25(5): 483-488. doi: 1002/uog.1879.
- Lim DJ, Park JY, Kim JH, et al. Clinical characteristics and outcome of hydronephrosis detected by prenatal ultrasonography. Journal of Korean Medical Science, 2003; 18(6): 859-862.
- Koff SA. Postnatal management of antenatal hydronephrosis using an observational approach. Urology, 2000: 55: 809-811. doi: 10.1016/s0090-4295(00)00459-3.

