Rare Cause of Pyrexia of Unknown Origin: Primary Gastrointestinal Non-Hodgkin’s Lymphoma
Aravinth A1,* and Dominic Rodriguez G2
1Department of Surgical Gastroenterology, Kauvery Hospital, Trichy, India
2Department of General Medicine, Kauvery Hospital, Trichy, India
Received Date: 15/09/2023; Published Date: 15/02/2024
*Corresponding author: Aravinth Anbarasu, Department of Surgical Gastroenterology, Kauvery Hospital, Trichy, India
Abstract
Background: Primary gastrointestinal non-Hodgkin’s lymphoma (PGINHL) is a rare lymphoproliferative disorder characterised by lymphomatous involvement of gastrointestinal tract in the absence of generalised lymphadenopathy, blood and bone marrow involvement. It can rarely present as intermittent fever without other localising features.
Case Presentation: We report the case of a 48-year-old man presenting with pyrexia of unknown origin (PUO) with vague lower abdominal pain. The diagnosis of PGINHL was made by contrast enhanced CT abdomen showing aneurysmal dilatation of distal ileum with multiple large perilesional lymphadenopathies and confirmed by FNAC. He was managed by multimodality treatment, Surgery-Enbloc resection of distal ileal lymphoma followed by adjuvant immunochemotherapy with steroids, rituximab, cyclophosphamide, vincristine, and doxorubicin (R-CHOP).
Conclusion: Our case illustrates that PGINHL can present as PUO and should be considered in the differential diagnosis of this syndrome, especially in the absence of infective foci. Surgical resection followed immunochemotherapy is the mainstay of treatment for PGINHL of small bowel.
Keywords: Pyrexia of Unknown origin; Primary Gastrointestinal non-Hodgkin’s lymphoma
Background
Pyrexia of unknown origin (PUO) can be a manifestation of occult malignancy. Lymphomas, that are solid malignancies of the lymphoid system, are rare but an important cause of PUO. The incidence of lymphoma continues to increase and has more than doubled in the past four decades. Primary GastroIntestinal Non-Hodgkin’s Lymphoma (PGINHL) is the commonest extranodal site of involvement in immunocompetant persons. PGINHL constitute 10–15% of all non-Hodgkin lymphomas (NHL) and 30–40% of all extranodal lymphomas [1].
In this report, the diagnosis and therapy for a rare case of primary small bowel lymphoma manifesting as PUO are presented and discussed in the light of the literature.
Case Presentation
A 48-year-old male presented to our Emergency Department with complaints of fever (high grade, intermittent, associated with chills and rigor) in the last two weeks. He also complained of generalised weakness and mild lower abdominal pain during this period. He reported cough with expectoration and dysuria in the last two days. He had loss of appetite and significant weight loss of about 10 kg over past five months. He was admitted and treated elsewhere with COVID-19 infection and associated Acute Limb Ischemia (bilateral femoropopliteal occlusion) five months back. Currently, on regular treatment with antiplatelet and anticoagulant drugs. Physical examination revealed pallor and mild tenderness in right iliac fossa. No organomegaly or significant lymphadenopathies were present. Chest and spine examination were normal.
Investigations:
Blood count showed the following: haemoglobin, 7.4g/dl; white cell count, 8,900 cells/cu mm and platelets, 3.18 lakhs/cu mm. The results of his renal and liver function tests were within normal limits. Inflammatory markers, Erythrocyte sedimentation rate and C-reactive protein were markedly elevated to 80 mm and 260.6 mg/L, respectively. Septic workup including blood and urine cultures was negative. Procalcitonin was normal (0.28 ng/ml). Chest X-ray was clear. Ultrasound abdomen revealed a 6 × 4.6 cm well defined heterogeneous mass with air pockets in right side of pelvis along with few enlarged iliac lymph nodes suggestive of abscess. Contrast enhanced CT scan of abdomen was done to further characterise the lesion. It showed a long concentric bowel wall thickening of distal ileum with surrounding bulky lymphadenopathy (Figure 1a and 1b).
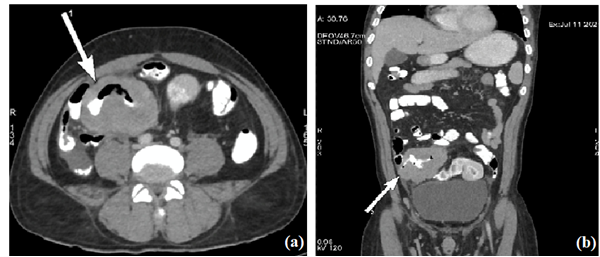
Figure 1a and 1b: CECT abdomen axial and coronal images respectively showing aneurysmal dilatation of distal ileum marked with arrow.
Differential diagnosis:
Differential diagnoses considered at this point were abdominal tuberculosis, small bowel lymphoma and a rather rare small bowel adenocarcinoma. Mantoux test was negative. Colonoscopy was done to obtain luminal biopsy from the lesion but the visualised terminal ileum was normal and distal ileal lesion couldn’t be accessed. Ultrasound guided trans-abdominal fine needle aspiration cytology (FNAC) did from the lesion revealed atypical lymphoid cells with high nuclear atypia, suggestive of lymphoma.
Treatment:
Patient was initially started on antipyretics along with empirical antibiotics meropenam and clindamycin. Severe iron deficiency with anaemia was supported with 2-unit blood transfusion and oral iron therapy. But after exclusion of infective aetiologies as mentioned above, intravenous antibiotics were stopped and only antipyretic on “si opus sit” (SOS) basis continued. In view of high suspicion of small bowel lymphoma, he underwent Exploratory Laparotomy. Intraoperatively there was large distal ileal tumour of size 6 × 5 cm about 30 cm from IC junction with large mesenteric lymph node deposits (Figure 2). Enbloc resection of distal ileal tumour with ileoascending anastomosis was done.
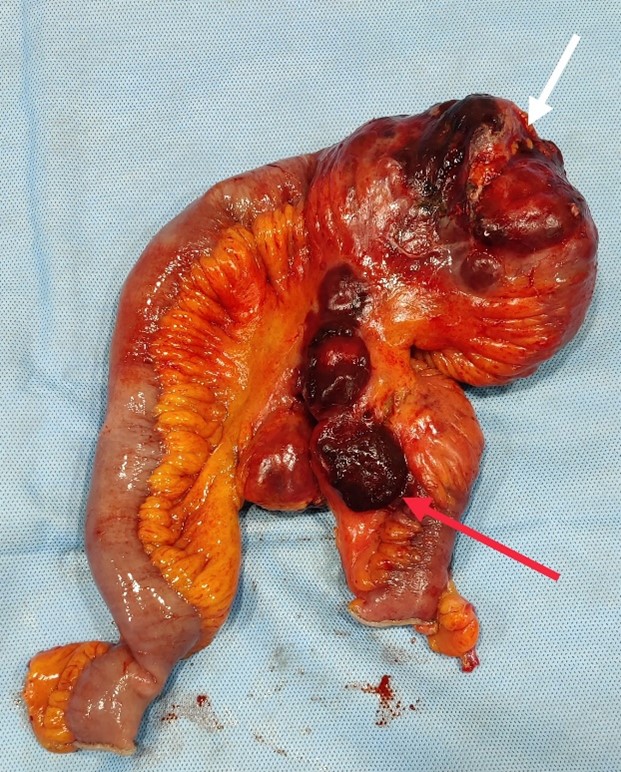
Figure 2: Post-surgery specimen showing large distal ileal tumour of size 6 × 5 cm (white arrow) about 30 cm from IC junction with large mesenteric lymph node deposits (red arrow).
Post-surgery histopathology report was suggestive of non-Hodgkin’s lymphoma. Microscopic examination showed large atypical lymphoid cells with scant cytoplasm and vesicular nuclei with distant nucleoli, classical of diffuse large B cell lymphoma (DLBCL) (Figure 3). Immunohistochemistry (IHC) was done to confirm and further type and grade the lymphoma. IHC findings were diffuse reactivity for CD20 (pan B cell antigen) with high Ki67 proliferation index of 95% (Figure 4a and 4b); also dim nuclear reactivity for transcription factor Multiple Myeloma 1(MUM1), suggestive of DLBCL non-germinal centre type (Figure 4c).

Figure 3: High power histopatological images showing monotonous infiltrate of large atypical lymphoid cells with scant cytoplasm and vesicular nuclei suggestive DLBCL.

Figure 4: a. IHC images showing diffuse reactivity for CD20 with (b) high Ki67 proliferation index of 95%. (c) Also dim nuclear reactivity for transcription factor MUM1, suggestive of DLBCL non-germinal centre type.
Outcome and follow-Up:
The patient was followed for one month after surgery. He is completely afebrile and doing well. He has started gaining weight, with no abdominal pain and obstructive symptoms like constipation/obstipation. Bone marrow examination was done to confirm PGINHL, which was normal. He was started on first cycle of adjuvant immunochemotherapy R-CHOP regimen.
Discussion
Pyrexia of Unknown Origin (PUO) was originally defined in 1961 by Petersdorf and Beeson as a disease condition of temperature exceeding 38.3 °C on at least three occasions over a period of at least three weeks, with no diagnosis made despite one week of inpatient investigation [2]. However, with modern diagnostic techniques and radiological advancements, many underlying diseases are reported for these classical PUO presentations and the definition constantly changes and it is still unresolved. PUO can be the symptom of over 200 different causes, and literature suggests classifying these aetiologies into five broad categories: infectious, neoplastic, non-infectious inflammatory, miscellaneous and undiagnosed conditions. Neoplasms are an important cause of PUO, accounting for about 7–31% of these cases [2]. Having high suspicion for neoplasm in PUO, helps to detect it at earlier stage and may enhance the prognosis after treatment.
Lymphomas are the second most common cause of PUO (12.5%), next only to tuberculosis (28.3%) in India[3]. PGINHL is the most common extra-nodal form of NHL, stomach being commonest gastrointestinal site followed by small bowel. Strict criteria were proposed by Dawson in 1961 to label as PGINHL, they are [4]:
- Absence of peripheral lymphadenopathy at the time of presentation
- Lack of enlarged mediastinal lymph nodes
- Normal total and differential white blood cell count
- Predominance of bowel lesion at the time of laparotomy with lymph nodes obviously affected only in the immediate vicinity
- No lymphomatous involvement of liver and spleen
PGINHL of small bowel differ from the more common gastric lymphoma in tumour characteristics and treatment practices. They are more heterogeneous than those in stomach include DLBCL; Enteropathy associated T-Cell Lymphoma (EATL), Mantle Cell Lymphoma (MCL), Follicular Lymphoma and Immunoproliferative Lymphoma (IPSID). Majority of the patient are treated with surgical resection followed by adjuvant chemotherapy except in IPSID and follicular lymphoma [5]. This may be due to the large number of patients diagnosed at the time of surgery and the 10–20 % of patients requiring emergency surgery due to their clinical presentation like perforation/obstruction. Lymphomas of small and large bowel rapidly melt away with appropriate chemotherapy resulting in intestinal perforation as they lack desmoplastic reaction commonly seen with adenocarcinomas [6]. Gastric lymphoma because of increased thickness of gastric wall and earlier diagnosis are resistant to this phenomenon and are commonly treated with chemotherapy with surgery as salvage treatment option. Multimodality treatment with surgery followed chemotherapy resulted in best Overall survival (OS) than surgery or chemotherapy alone (91 vs. 62%), but this difference becomes less pronounced as the stage of tumour advances [5].
Learning points
- Treating physicians should always have strong suspicion for underlying undiagnosed malignancy as cause of fever while evaluating the aetiology of pyrexia of unknown origin.
- Surgical resection followed immunochemotherapy is the mainstay of treatment for PGINHL of small bowel.
Acknowledgements: Thankful to Dr. R. Subbaiah, Consultant Hematooncologist and Drs. K. Kavitha and M. Anitha, Pathologists, Neuberg-Ehrilich Laboratory.
References
- Cardona DM, Layne A, Lagoo AS. Lymphomas of the gastro-intestinal tract - Pathophysiology, pathology, and differential diagnosis. Indian J Pathol Microbiol, 2012; 55(1): 1.
- Unger M, Karanikas G, Kerschbaumer A, et al. Fever of unknown origin (FUO) revised. Wien Klin Wochenschr, 2016; 128: 796–801.
- Pannu AK, Golla R, Kumari S, et al. Aetiology of pyrexia of unknown origin in north India: Trop Doct, 2020; 51(1): 34-40.
- Ghimire P, Wu G-Y, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol, 2011; 17(6): 697–707.
- Lightner AL, Shannon E, Gibbons MM, et al. Primary gastrointestinal non-Hodgkin’s lymphoma of the small and large intestines: a systematic review. J Gastrointest Surg, 2015; 20(4): 827–839.
- Tatar C, Yavas M, Akkus O, et al. Intestinal perforation that developed after chemotherapy in a patient diagnosed with non-Hodgkin lymphoma: A case report and review of literature. Int J Surg Case Rep, 2017; 39: 321–323.

