A Clinical Case Report on the Pleomorphic Adenoma of the Parotid Gland
Peter F Cammans1, Eraj Sattar2, Aysha Hassan2, Zafar Qureshi3,*, Zoya H. Rizvi4 and Syed AA Rizvi5,*
1Dr Kiran C Patel College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, Florida, USA
2Florida International University, Miami, Florida, USA
3UMC Free Clinic, Miami Gardens, Florida, USA
4Adlai E. Stevenson High School, Lincolnshire, Illinois, USA
5College of Biomedical Sciences, Larkin University, Miami, Florida, USA
Received Date: 01/06/2023; Published Date: 02/10/2023
*Corresponding author: Syed A. A. Rizvi, MD, PhD, MPH, MBA, College of Biomedical Sciences, Larkin University, 18301 N Miami Ave, Miami, FL 33169, USA.
Zafar Qureshi, MD, UMC Free Clinic, Miami Gardens, Florida, USA
Abstract
A 68-year-old African-American male from Nigeria with a past medical history of hypertension, diabetes, and pleomorphic adenoma of the parotid gland, presented to clinic for reoccurrence of a painless, enlarging right facial mass with a history of two prior resections, occurring in 2012 and 2014 respectively. The CT indicted that the mass is medially displacing the right carotid artery and internal jugular vein. The mas was later removed, and the pathology report confirmed pleomorphic adenoma and negative for tumor.
Introduction
The Parotid Gland (PG) is the largest of exocrine glands located in the head and neck region that makes and secrete saliva, which plays a crucial role in the digestion of food. it is situated on both sides of the face, in front of the ear extending from the temple area down to the angle of the jaw. The parotid gland contributes the largest volume of saliva among the salivary glands. The parotid gland is drained by the parotid duct, also known as Stensen's duct [1].
The parotid gland can be affected by various conditions, including both benign and malignant tumors. The most common benign tumor is pleomorphic adenoma, while the most common malignant tumor is mucoepidermoid carcinoma. Other conditions that can affect the parotid gland include infections, salivary gland stones (sialolithiasis), and autoimmune disorders such as Sjögren's syndrome [2,3].
Surgical procedures involving the parotid gland are often performed to remove tumors or manage conditions that do not respond to conservative treatment. The surgical approach may vary depending on the size, location, and nature of the condition. Common surgical techniques include superficial parotidectomy or total parotidectomy [4]. The facial nerve, also known as the seventh cranial nerve, courses through the parotid gland. It controls the muscles of facial expression. During parotid gland surgery, great care must be and is taken to preserve the integrity of the facial nerve and maintain facial function [5].
Case Presentation
The patient stated that he first noticed the mass in 2008, and subsequently had it resected in Nigeria in 2012. He then stated that over the next two years the mass slowly grew back, so he underwent a second resection in Nigeria in 2014. In 2018, while visiting his son in Florida, he underwent Fine Needle Aspiration (FNA) of the mass at the University of Miami Hospital and Clinics. The patient then stated that the resulting pathology report showed the mass was benign, and due to financial reasons, he elected to forgo surgery at the time. During review of systems, the patient denied having any fever, chills, night sweats, weight loss, headaches, ear/throat pain, voice changes, or swallowing difficulty. He also denied ever using tobacco, alcohol, or drugs. Lastly, the patient reported no family history of head/neck cancer, skin cancer, or history of radiation exposure.
On examination, the patient appeared healthy, well-nourished, and well-developed. His height was 167 cm (66 in) and his weight was 83 kg (183 lbs), with a corresponding body mass index of 29.5 kg/m2. He was hypertensive, with a blood pressure in the right arm of 165/96 mmHg while sitting, but all other vital signs were within normal limits. He was alert and oriented to person, place, and time. His pupils were equal, round, and reactive to light and accommodation. Extraocular movements were full and intact, and conjunctivae were clear bilaterally. Inspection of the head revealed a large, right-sided facial mass with irregular borders (Figure 1). Findings of lung, heart, abdomen, and skin examinations were unremarkable.
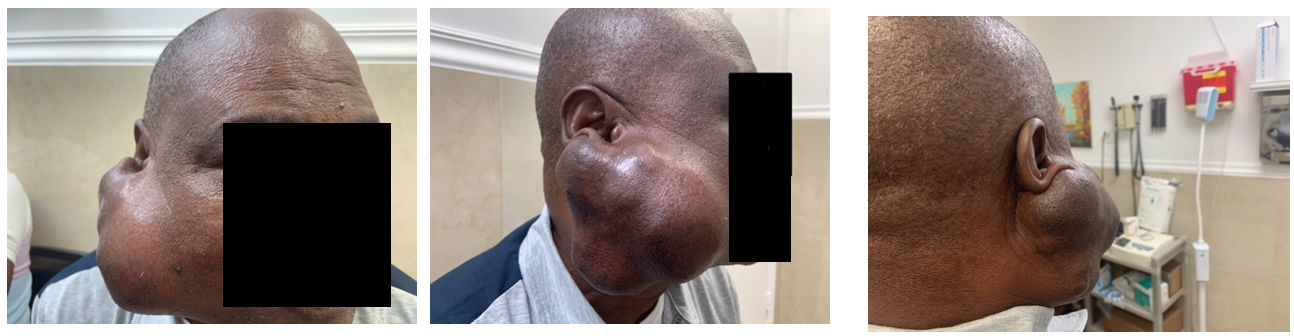
Figure 1: Anterior, Lateral, and Posterior views of the painless, enlarging right-sided facial mass.
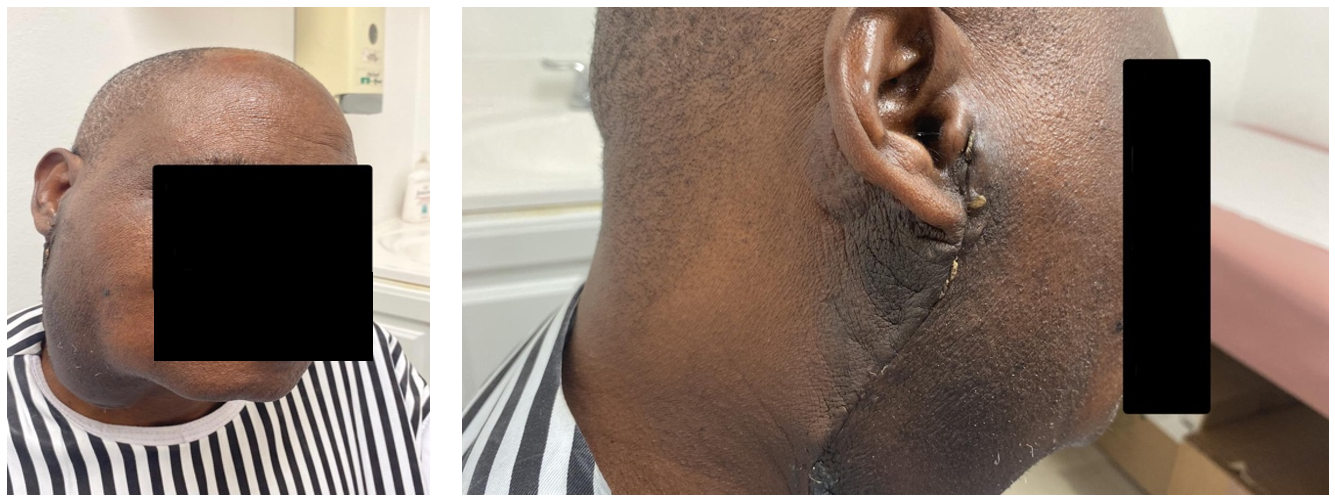
Figure 2. Post-surgical Anterior and Lateral views.
Prior FNA cytology report, completed in 2018 at the University of Miami Hospital and Clinics (UMHC), revealed atypical epithelial cells present in a background of pleomorphic adenoma, however, carcinoma could not be excluded with certainty. Prior Computed Tomography (CT) scan of the neck with IV contrast, also completed in 2018 at UMHC, showed a 7.5-cm x 7.5-cm x 8.0-cm multilobulated tumor in the right parotid gland extending towards the parapharyngeal space (PPS) in the neck, and severe right lung consolidation with biapical thickening.
Laboratory test results, completed two days after initial visit included the following values: Hemoglobin A1C, 11.5% (reference range, <5.7%); Fasting Glucose, 338 mg/dL (reference range, 65-99 mg/dL); Blood Urea Nitrogen (BUN), 22 mg/dL (reference range, 7-25 mg/dL); Creatinine, 1.48 (reference range, 0.70-1.25 mg/dL); Estimated Glomerular Filtration Rate (eGFR) for an African-American, 56 mL/min/1.73m2 (reference range, >60 mL/min/1.73m2); Red Blood Cell Count, 6.33 million/uL (reference range, 4.20-5.80 million/uL); Hemoglobin, 13.6 g/dL (reference range, 13.2-17.1 g/dL); Hematocrit, 46.5% (reference range, 38.5-50.0%); Mean Corpuscular Volume (MCV), 73.5 fL (reference range, 80.0-100.0 fL); Mean Corpuscular Hemoglobin (MCH), 21.5 pg (reference range, 27.0-33.0 pg); Mean Corpuscular Hemoglobin Concentration (MCHC), 29.2 g/dL (reference range, 32.0-36.0 g/dL); Total Cholesterol, 185 mg/dL (reference range, <200 mg/dL); Low Density Lipoprotein (LDL)-Cholesterol, 107 mg/dL (reference range, 70-100 mg/dL); High Density Lipoprotein (HDL), 31 mg/dL (reference range, >40 mg/dL); Cholesterol/HDL Ratio, 6.0 (reference range, <5); Non-HDL Cholesterol, 154 mg/dL (reference range, <130 mg/dL).
A new CT scan of the neck with IV contrast was then obtained, the results of which showed a large soft tissue mass in the right cervical soft tissues measuring 9-cm x 10-cm x 10-cm. The epicenter of the mass was found to be in the superficial deep lobe of the right parotid gland and was found to be abutting the masseter muscle as well as the sternocleidomastoid muscle and right submandibular gland. Additionally, the CT scan showed the mass to be medially displacing the right carotid artery and internal jugular vein.
Additionally, biapical-pleural thickening was noted, with the right apex being worse, and bronchiectactic changes noted in the right apex. Biopsy/resection of the parotid mass was recommended. The subsequent pathology report acquired after resection of the mass reported: Pleomorphic adenoma, 8.5-cm, with satellite foci in the dermis of the overlying skin. Specimen margins are negative for tumor. Five benign lymph nodes.
Discussion
Pleomorphic adenomas (PA), also known as benign mixed tumor, is the most common tumor of the salivary glands, particularly the parotid gland. It is a slow-growing neoplasm that arises from the epithelial cells and myoepithelial cells within the salivary gland tissue. Pleomorphic adenoma is characterized by its diverse histological appearance and is composed of a mixture of different cell types [6,7]. Pleomorphic adenoma is the most common salivary gland tumor, accounting for approximately 60-70% of all benign salivary gland tumors [8]. The parotid gland is the most commonly affected site, followed by the minor salivary glands [9]. Most patients with pleomorphic adenoma present with a painless, slowly growing mass in the parotid gland region. The tumor is usually mobile and well-defined, and it may cause facial asymmetry. In some cases, there may be facial nerve involvement, leading to symptoms such as facial weakness or paralysis [10].
Pleomorphic adenoma is characterized by a mixture of epithelial and myoepithelial cells arranged in various patterns, including glandular, trabecular, tubular, and cribriform structures. The stroma within the tumor is often myxoid or hyalinized. The presence of chondromyxoid stroma is a characteristic feature of pleomorphic adenoma [11]. Although pleomorphic adenoma is benign, it has a potential for local recurrence if not completely removed. Recurrence is more common in tumors that have been incompletely excised or if there has been rupture of the tumor capsule during surgery. Rarely, pleomorphic adenoma can undergo malignant transformation into carcinoma ex pleomorphic adenoma [12,13].
The diagnosis of pleomorphic adenoma is made through a combination of clinical examination, imaging studies (such as ultrasound, CT scan, or MRI), and a fine-needle aspiration biopsy. However, definitive diagnosis is usually achieved through surgical excision and subsequent histopathological examination [14]. The primary treatment for pleomorphic adenoma is surgical excision. The goal is complete removal of the tumor while preserving the facial nerve function. Depending on the size and location of the tumor, different surgical approaches may be used, including superficial parotidectomy, total parotidectomy, or even minimally invasive techniques. Radiation therapy may be considered in cases of recurrent or unresectable tumors [15-17].
Pleomorphic adenoma has an overall favorable prognosis, with a low incidence of malignant transformation. The long-term survival rate is excellent if the tumor is completely excised. However, recurrence rates vary depending on factors such as the completeness of initial resection, tumor size, and histological subtype.
Disclosures: The authors report no relevant financial relationships.
References
- Ghannam MG, Singh P. Anatomy, Head and Neck, Salivary Glands. [Updated 2022 Jun 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023.
- Geiger JL, Ismaila N, Beadle B, et al. Management of Salivary Gland Malignancy: ASCO Guideline. J Clin Oncol, 2021; 39(17): 1909-1941. doi:10.1200/JCO.21.00449.
- Krishnamurthy S, Vasudeva SB, Vijayasarathy S. Salivary gland disorders: A comprehensive review. World J Stomatol, 2015; 4(2): 56-71. DOI: https://dx.doi.org/10.5321/wjs.v4.i2.56.
- Psychogios G, Bohr C, Constantinidis J, et al. Review of surgical techniques and guide for decision making in the treatment of benign parotid tumors. Eur Arch Otorhinolaryngol. 2021;278(1):15-29. doi:10.1007/s00405-020-06250-x.
- Chason HM, Downs BW. Anatomy, Head and Neck, Parotid Gland. [Updated 2022 Oct 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2023.
- Bokhari MR, Greene J. Pleomorphic Adenoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Abdelhamid AS, Elzayat S, Essa AA, et al. Pleomorphic adenoma of the cheek: a case presentation. Egypt J Otolaryngol, 2022; 38: 165. https://doi.org/10.1186/s43163-022-00352-5.
- Young A, Okuyemi OT. Benign Salivary Gland Tumors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Alsanie I, Rajab S, Cottom H, et al. Distribution and Frequency of Salivary Gland Tumours: An International Multicenter Study. Head Neck Pathol, 2022; 16(4): 1043-1054. doi:10.1007/s12105-022-01459-0.
- Jain S, Hasan S, Vyas N, Shah N, Dalal S. Pleomorphic Adenoma of the Parotid Gland: Report of a Case With Review of Literature. Ethiop J Health Sci, 2015; 25(2): 189-94. doi: 10.4314/ejhs.v25i2.13.
- Radhika T, Maheswari SU, Kumar KS, Jeddy N. Rare histologic presentation of pleomorphic adenoma: A diagnostic dilemma. J Oral Maxillofac Pathol, 2020; 24(3): 563-567. doi: 10.4103/jomfp.JOMFP_62_20.
- Aro K, Valle J, Tarkkanen J, Mäkitie A, Atula T. Repeatedly recurring pleomorphic adenoma: a therapeutic challenge. Acta Otorhinolaryngol Ital, 2019; 39(3): 156-161. doi: 10.14639/0392-100X-2307.
- Grasso M, Fusconi M, Cialente F, de Soccio G, Ralli M, Minni A, et al. Rupture of the Pleomorphic Adenoma of the Parotid Gland: What to Know before, during and after Surgery. J Clin Med, 2021; 10(22): 5368. doi: 10.3390/jcm10225368.
- Bussu F, Parrilla C, Rizzo D, Almadori G, Paludetti G, Galli J. Clinical approach and treatment of benign and malignant parotid masses, personal experience. Acta Otorhinolaryngol Ital, 2011; 31(3): 135-143.
- Schapher M, Koch M, Goncalves M, Mantsopoulos K, Iro H. Extracapsular Dissection in Pleomorphic Adenomas of the Parotid Gland: Results After 13 Years of Follow-up. Laryngoscope, 2021; 131(2): E445-E451. doi:10.1002/lary.28696.
- Quer M, Hernandez-Prera JC, Silver CE, et al. Current Trends and Controversies in the Management of Warthin Tumor of the Parotid Gland. Diagnostics (Basel), 2021; 11(8): 1467. doi:10.3390/diagnostics11081467.
- Thielker J, Grosheva M, Ihrler S, Wittig A, Guntinas-Lichius O. Contemporary Management of Benign and Malignant Parotid Tumors. Front Surg, 2018; 5: 39. doi:10.3389/fsurg.2018.00039.

