Pancreatic Artery Pseudoaneurysm complicated by superimposed COVID-19 Infection, Atrial Fibrillation and Acute Delirium
Larissa Check,*, Anna Saldanha, Mary Carter and Chadley Froes
Department of Internal Medicine. Grand Strand Medical Center, Myrtle Beach, SC, USA
Department of Anesthesiology. Grand Strand Medical Center, Myrtle Beach, SC, USA
Received Date: 30/04/2022; Published Date: 30/05/2022
*Corresponding author: Larissa Check, Department of Internal Medicine. Grand Strand Medical Center, Myrtle Beach, SC, USA, Email: Larissa.check@hcahealthcare.com
Abstract
Pancreatic artery pseudoaneurysms are a relatively rare outpouching of the vascular wall that occurs following vascular trauma, leading to hematoma formation and subsequent dilation of the surrounding portion of the arterial wall. They may occur in the setting of chronic pancreatitis, where inflammation leads to the release of pancreatic enzymes that are thought to erode the vessel, leading to hematoma and dilation. Unlike true aneurysms that contain the normal trilaminar histological structure of normal arterial walls, pseudoaneurysms may lack one or more histological layers of the vascular tunica. They most commonly occur in the splenic artery, but can also occur in other portions of pancreatic arterial blood supply as a complication of chronic inflammation of the pancreas or through direct mechanical trauma. We present a case involving a 72-year-old male with a history of chronic pancreatitis, alcohol abuse, diabetes and hyperlipidemia who presented with abdominal and rib pain with associated nausea following trauma sustained during a brawl with another individual. Physical examination was remarkable for abdominal distension and severe tenderness to palpation. The patient underwent CT angiography which revealed pancreatic pseudoaneurysm involving his gastroduodenal artery. Interventional radiology intended to perform an embolization of the pseudoaneurysm, however the patient soon became delirious secondary to acute alcohol withdrawal and tested positive for COVID-19 on PCR. Furthermore, telemetry monitoring was noted for atrial fibrillation with rapid ventricular response, likely driven by the autonomic hyperactivity secondary to alcohol withdrawal. Given the atrial arrhythmia concomitant COVID19 infection, anticoagulation was initiated. However, we remained cautious regarding the possibility of expanding the pseudoaneurysm, given the compromised structure of the vessel wall. As such, we present a difficult management of a rare but fatal vascular complication of chronic pancreatitis. Our case illustrates the importance of prompt recognition and management, while demonstrating management decisions to prevent further complications, many of which carry a substantial risk of morbidity and mortality.
Keywords: Pancreatic artery pseudoaneurysm; Rare complication of Covid-19; Newly detected atrial fibrillation; Alcohol withdrawal
Introduction
A true aneurysm is a permanent dilatation of all three layers of the artery—the tunica intima, media and adventitia. Pseudoaneurysms or “false aneurysms”, only involve the intimal and medial layers of the artery. A pancreatic artery pseudoaneurysm is a rare but potentially fatal complication classically associated with chronic pancreatitis. Pseudoaneurysms in general occur in approximately 3.5% - 10.5% of cases of pancreatitis, and carry a mortality as high as 50% [1]. The most common locations of pseudoaneurysm associated with pancreatitis involve the splenic artery, gastroduodenal and pancreaticoduodenal artery [2]. In the setting of chronic pancreatitis, pseudoaneurysms form as a result of auto-digestion of the vessel wall by pancreatic enzymes.
Weakening of the artery leaves it vulnerable to further malformation, especially if there is superimposed trauma to the abdomen. The pseudoaneurysm can enlarge into a hematoma which can rupture into the peritoneal cavity [2]. Clinical presentation of this vascular malformation is often nonspecific and is usually diagnosed following incidental visualization on imaging. The most common symptom is abdominal pain and this usually reflects an underlying acute or chronic pancreatitis [3]. If left untreated however, a pancreatic artery pseudoaneurysm can result in life-threatening hemorrhage and hemodynamic instability [2,3].
Case Presentation
A 72-year-old male with a past medical history significant for chronic pancreatitis, alcohol abuse, diabetes, and hyperlipidemia were admitted as a transfer from an Outside Hospital (OSH) after incidental finding of a vascular malformation of the pancreatic artery on CT imaging of the abdomen and pelvis. Upon admission, the patient endorsed generalized weakness, malaise, severe abdominal pain, and nausea. Prior to this admission, he was previously admitted for an acute-on-chronic pancreatitis and discharged after three days of inpatient treatment. Upon discharge, the patient was involved in a physical altercation, during which he alleged he was hit in the abdomen. He was readmitted to the same, aforementioned OSH wherein CT imaging demonstrated a new vascular malformation. He was then transferred to our hospital for intervention. Physical exam was remarkable for a bruise on the left side of his abdomen and diffuse abdominal tenderness to palpation. CTA Abdomen and Pelvis with contrast revealed a 1.6cm pseudoaneurysm at the pancreatic head and involving the gastroduodenal artery. There was no evidence of retroperitoneal hemorrhage. Interventional radiology agreed to perform an angioembolization procedure. As part of the pre-procedure workup, a COVID antigen and PCR test were performed. Both tests were positive, however, the patient was asymptomatic and initial vitals were unremarkable. Chest radiograph was unremarkable for an acute intrathoracic process. CBC was remarkable for Hgb and Hct of 10.3 g/dl and 30.6 L/L respectively, MCV>100 fL; CMP was unremarkable and Lipase was elevated at 657 U/L.
Given the patient’s stability and high risk of rupture, we planned for mesenteric angiography with embolization the next day. However, the patient’s course was complicated by acute withdrawal from alcohol with symptoms of agitation, delirium and hallucination. As a result of this acute delirium, he was no longer a safe candidate for arterial embolization until delirium resolved. Furthermore, he became tachycardic and transitioned from sinus tachycardia to atrial fibrillation with rapid ventricular response. This was even further complicated by acute respiratory distress with oxygen saturations dropping into the low 90s. Repeat imaging of the chest showed new infiltrates which were concerning for the development of pneumonia of either typical or atypical nature (including COVID-19). The patient was started on antibiotics, steroids and anticoagulation. A beta blocker was also initiated for rate control of the atrial fibrillation. He was not a candidate for experimental use of remdesivir or convalescent plasma for COVID-19 pneumonia. Complete blood count was closely monitored for acute blood loss anemia as this could drive the tachycardia and also cause hemodynamic instability. A repeat CTA of Abdomen and Pelvis with contrast revealed a slight increase in the size of pseudoaneurysm to 2.5 x 2.1cm. On the third day, the patient’s delirium resolved and the decision was made to proceed with the embolization procedure. Through the right common femoral artery, a microcatheter technique was used to confirm and embolize a pseudoaneurysm in the pancreatic head which appeared to be off of the posterior division of the superior pancreaticoduodenal arcade. Completion rteriogram demonstrated patency of the majority of the branches with continuous flow into the gastroepiploic artery.
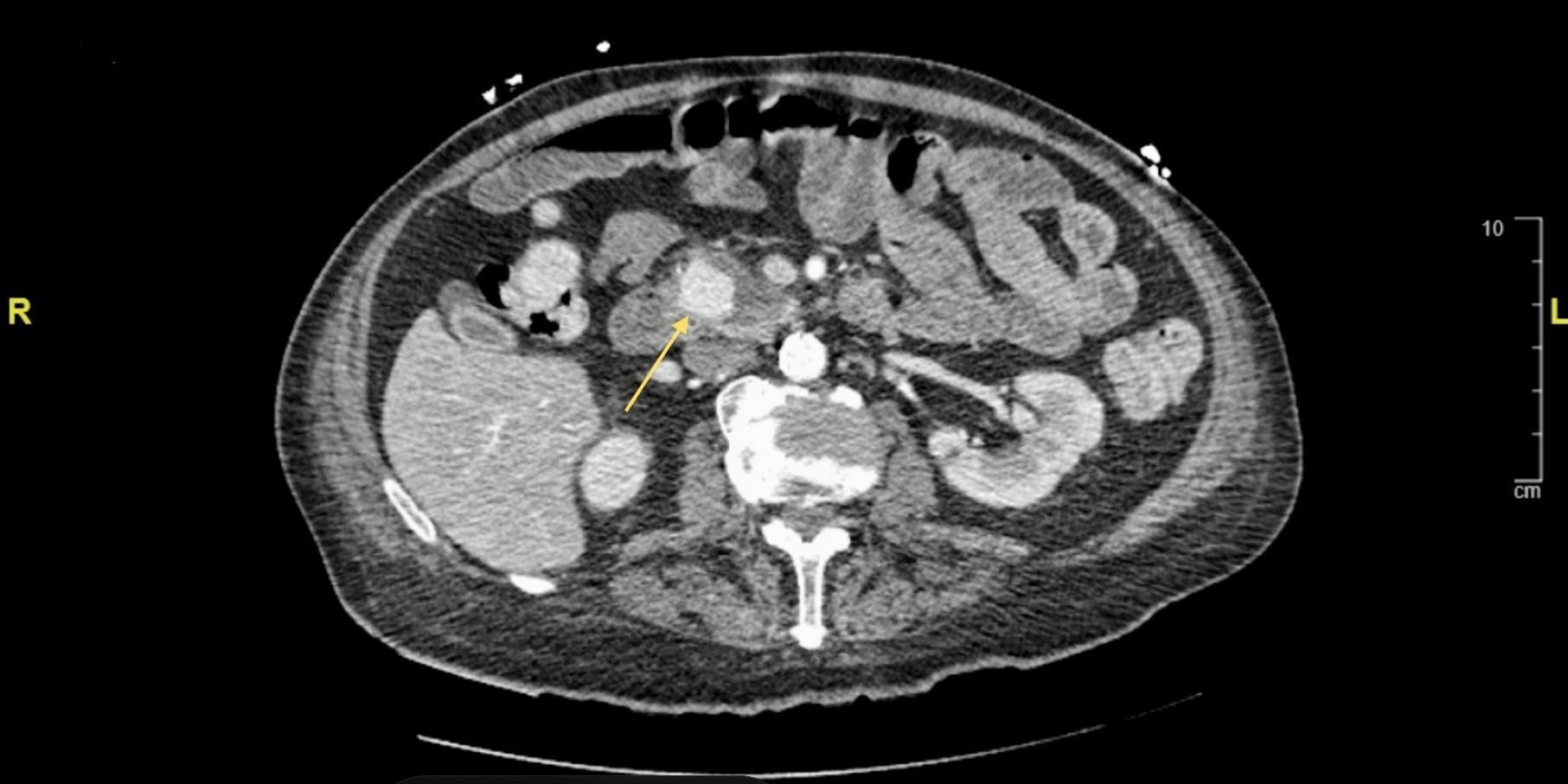
Figure 1: Contrast enhanced Computed tomography showing axial cut of a 2.5 x 2.1 cm posterior pancreaticoduodenal pseudoaneurysm (yellow arrow), a branch of the gastroduodenal artery.
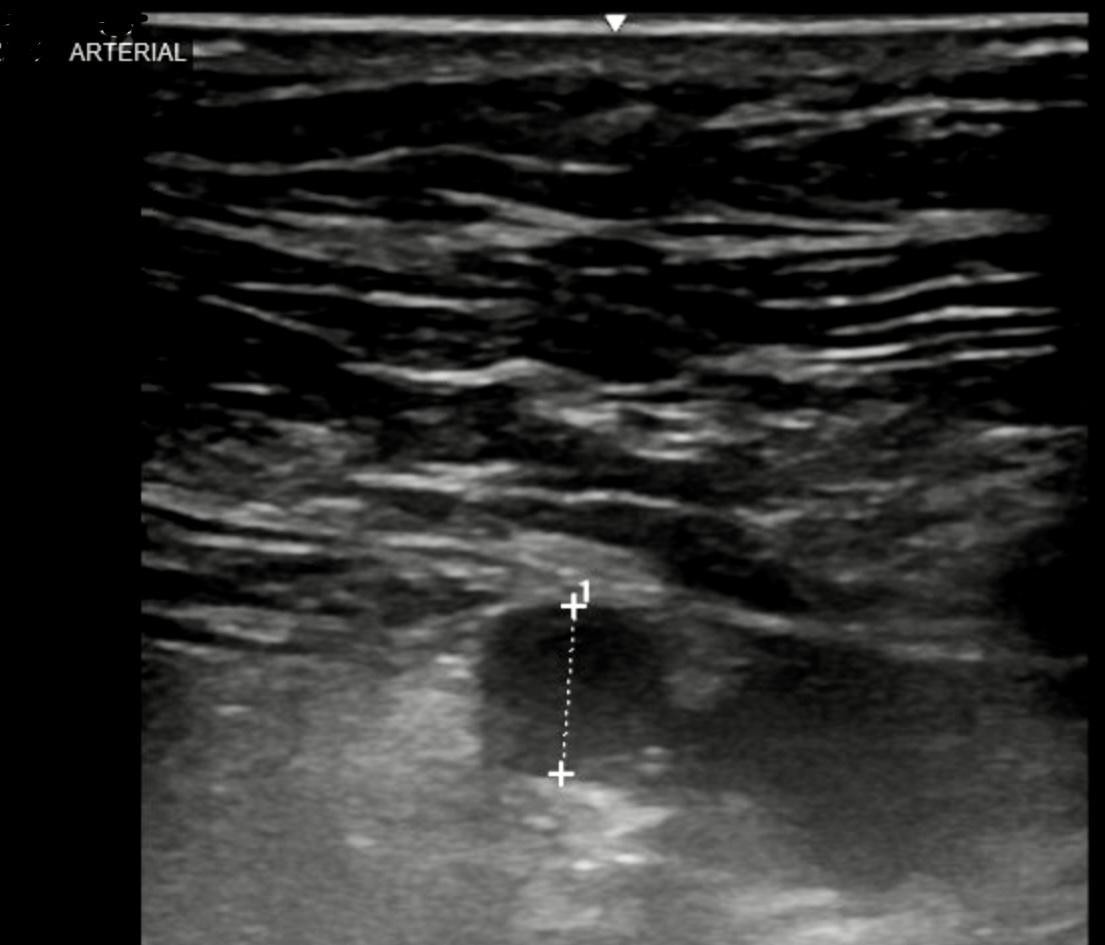
Figure 2: Arterial ultrasound taken prior to embolization procedure. It showed the 2.5 x 2.1 cm pseudoaneurysm.

Figure 3: Angiography and endovascular repair of the pseudoaneurysm. The posterior division of the pancreaticoduodenal artery was selected distal to the aneurysm which was then embolized to stasis with the 2 mm micro coils. Pullback arteriogram as the coils were deposited confirmed occlusion of the origin of the pseudoaneurysm. Completion arteriogram demonstrated patency of the majority of the branches with continuous flow into the gastroepiploic artery.
Discussion
Pancreatic pseudoaneurysms occur most commonly in the setting of recurrent pancreatitis wherein digestive pancreatic enzymes erode the pancreatic or other peripancreatic arteries [4]. The resulting pathology is a fragile, contained hematoma where the surrounding wall consists of produts of the clotting cascade, including fibrin and platelet crosslinks that are substantially weaker than the normal vascular structure [5,6]. Pseudoaneurysms can also occur in the setting of mechanical damage to the vasculature, typically following pancreaticobiliary surgery, transplantation, or trauma [4]. Our patient’s pseudoaneurysm was diagnosed when he presented to the hospital following blunt abdominal trauma sustained during a physical altercation, after he was discharged for an acute exacerbation of pancreatitis. His presenting symptoms were significant for nausea, right upper quadrant pain, epigastric pain, and left upper quadrant pain.
The statistical frequency of gastroduodenal pseudoaneurysms, like our patient’s, is 10-20% of all reported peripancreatic pseudoaneurysms and 1.5% of all reported visceral arterial pseudoaneurysms [7,8]. Pseudoaneurysm only occurs in 3.5-10.5% of pancreatitis cases in general [1]. Diagnosis of pancreatic pseudoaneurysms is typically made through incidental findings on abdominal or pelvic CT imaging; this modality is 67% sensitive for diagnosing a pancreatic pseudoaneurysm [9]. Literature is consistent in affirming CT angiography as the gold standard diagnostic modality for pancreatic pseudoaneurysms with the greatest sensitivity of 100% [9]. Less sensitive modalities include ultrasonography (50%) and upper endoscopy (20%) [9].
Gurala, D., et al. proposed a classification system based on the relative size of the originating rtery (including the capacity for surgical excision), communication to the gastrointestinal tract (GIT), and on the presence or absence of pancreatic juices [10]. Specifically, type I psuedoaneurysms occur in minor arteries that can be easily excised, while type II occurs in major arteries with minimal hemodynamic compromise if surgically removed. Type III are not amenable to surgical removal given their association with major arteries that cannot be excised. Further subclassification depends on the presence or absence of communication with the GIT. Type A pseudoaneurysms lack communication, and tend to produce lower volumes of hemorrhage leading to a confined hematoma. Note that with expansion into the retroperitoneal space, these can compress the bleeding site, leading to abdominal compartment syndrome. On the other hand, type B pseudoaneurysms maintain communications with the GIT, which tend to begin as minor, localized bleeding but are more likely to progress to fatal hemorrhage. Lastly, they are categorized based on the presence or absence of pancreatic juices, with implications regarding the likelihood of successful stent placement and viability. Type 1 pseudoaneurysms lack pancreatic secretions and are typically excellent candidates for endovascular stenting or embolization. Type 2 pseudoaneurysms are exposed to pancreatic juices, imparting a greater risk of enzymatic degradation including potential destruction of the stent or embolization agent, making endovascular approaches less viable. Ultimately, classification aims to simplify and guide management decisions on the basis of characteristics that may preclude specific management trajectories. Our patient’s pseudoaneurysm involved the gastroduodenal artery without communication to the GIT and with no evidence of pancreatic juices, and was thus classified as type IIA1. While both surgical and endovascular approaches were feasible courses of management in a hemodynamically stable patient, embolization would be less invasive and was performed on our patient in an effort to minimize the risk of surgical complications.
Conclusion
Pseudoaneurysms are life-threatening complications of chronic pancreatitis that carry a high mortality index. It is well-known that early identification and treatment is life-saving. These pseudoaneurysms are also at high risk of rupturing and bleeding. We present a complex case wherein a patient with a pancreatic pseudoaneurysm required urgent embolization while also challenging our management decision on anticoagulation as he also presented with atrial fibrillation and the novel COVID-19 infection. Furthermore, the patient experienced acute delirium which compromised his ability to cooperate and tolerate the embolization procedure.
All of these elements made every decision complex and critical. We prioritized arterial embolization of the pseudoaneurysm as soon as the patient was no longer delirious and this was completed successfully. We further discussed the risks and benefits of anticoagulation with the patient’s medical decision maker and agreed on the shared decision of accepting the risk of bleeding for the benefit of stroke prevention. Our patient received a CTA Abdomen and Pelvis prior to discharge which showed complete resolution of the pseudoaneurysm. This case highlights angio-embolization and endovascular stenting as the gold standard of treatment for pseudoaneurysms. It further emphasizes the need for prompt treatment even in the setting of multiple severe illnesses.
Disclosures: None
Author contributions: All authors contributed equally to this manuscript. L.Check is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- Lendrum R. Chronic pancreatitis. In: Misiewicz JJ, Pounder RE, Venables CW, eds. Diseases of the gut and pancreas. London: Blackwell Scientific Publications, 1994; 441- 454.
- Boudghene F, L’Hermine C, Bigot JM. Arterial complications of pancreatitis: Diagnostic and therapeutic aspects in 104 cases. J. Vasc. Interv. Radiol; 1993; 4: 551–558. doi: 10.1016/S1051-0443(93)71920-X.
- Mallick B, Malik S, Gupta P, Gorsi U, Kochhar S, Gupta V, et al. Arterial pseudoaneurysms in acute and chronic pancreatitis: Clinical profile and outcome. JGH Open, 2019; 3: 126–132. doi: 10.1002/jgh3.12116.
- Gurala D, Polavarapu AD, Idiculla PS, Daoud M, Gumaste V. Pancreatic Pseudoaneurysm from a Gastroduodenal Artery. Case Rep Gastroenterol, 2019; 13: 450- 455. doi: 10.1159/000503895
- Hoilat GJ, Mathew G, Ahmad H. Pancreatic Pseudoaneurysm. [Updated 2021 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- Rivera PA, Dattilo JB. Pseudoaneurysm. [Updated 2021 Mar 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
- A. Volpi, E. Voliovici, F. Pinato et al., “Pseudoaneurysm of the gastroduodenal artery secondary to chronic pancreatitis,” Annals of Vascular Surgery, 2010; 24(8): pp. 1136.e7–1136.e11.
- Nemakayala Divyesh, Ling Xiao, Laird-Fick Heather. Gastroduodenal Artery Pseudoaneurysm: A Complication of Pancreatitis, American Journal of Gastroenterology, 2017; 112; p S689.
- Harris K, Chalhoub M, Koirala A. Gastroduodenal artery aneurysm rupture in hospitalized patients: An overlooked diagnosis. World journal of gastrointestinal surgery, 2010; 2(9); 291–294.
- Pang TC, Maher R, Gananadha S, Hugh TJ, Samra JS. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc, 2014; 28(7): 2027-2038. doi: 10.1007/s00464-014-3434-9. Epub 2014 Feb 12. PMID: 24519028; PMCID: PMC4065337.

