Intradiverticular Ampullary Lesion as a Rare Cause of Bacteremia on an Hemodialysed Patient
Soraia Proença e Silva1,*, João Grilo2, Marco Pereira3, Joana Coutinho4, Ernesto Rocha5
1Internal Medicine Resident, Hospital Amato Lusitano, Portugal
2Nephrology Resident, Hospital Amato Lusitano, Portugal
3Gastroenterology Resident, Hospital Amato Lusitano, Portugal
4Nephrolog Assistant, Hospital Amato Lusitano, Portugal
5Nephrology Assistant and chief of Nephrology Department, Hospital Amato Lusitano, Portugal
Received Date: 28/02/2022; Published Date: 10/03/2022
*Corresponding author: Soraia Andreia Proença e Silva, Internal Medicine Resident, Hospital Amato Lusitano, Castelo Branco, Portugal
Abstract
An ampullary lesion localizes in the ampulla of Vater and an intraluminal duodenal diverticulum is a rare congenital malformation. An ampullary lesion can mobilize into the interior of such diverticulum favoring cholestasis by blocking the main biliary duct and infrequently results in acute cholangitis.
We report a rare case of a 69-year-old male on hemodialysis due to autosomal dominant polycystic kidney disease that was hospitalized at the nephrology department for the 5th time in one year period. On the first 4 hospitalizations the patient had presented with fever of unknown origin and was diagnosed sepsis secondary to broad-spectrum beta-lactamase producer Escherichia coli (E.coli) bacteremia however the exact origin of the fever was not identified. On the 5th hospitalization he presented with acute cholangitis allowing final diagnosis of underlying cause.
Endoscopic study identified a villous lesion prolapsing into an intraluminal duodenal diverticulum corresponding to the ampulla of Vater. Duodenoscopy allowed extrusion of the lesion from the diverticulum and biopsy revealed a tubular adenoma with low-grade dysplasia. The case describes an ampullary lesion hidden into an ampullary diverticulum, which despite being a benign lesion, was causing recurrent bacteremia due to intestinal translocation in a hemodialysis patient. The reported case presents an infrequent presentation for ampullary lesions and posed great difficulties not only for diagnosis but also in treatment due to its infectious and recurrent character. We hope that it may be of educational value to both physicians and surgeons alike.
Keywords: Recurrent fever; Ampullary lesion; Intraluminal duodenal diverticulum; Cholangitis; Autossomal dominant polycystic kidney disease
Introduction
Any lesion located in the ampulla of Vater is called an ampuloma. An Intraluminal Duodenal Diverticulum (IDD) is a rare congenital malformation that usually appears in the 2nd portion of the duodenum (DII), close to the ampulla of Vater. An IDD is formed through a diaphragm of the duodenal mucosa that protrudes into the lumen of the duodenum in the form of a sac and its appearance resembles that of an invagination. An ampuloma can favor the phenomena of cholestasis if mobilized into the insides of an intra duodenal diverticulum and obstructs the common bile duct [1-3]. Infrequently it can result in acute cholangitis based on two main phenomena: obstruction of the biliary tract by an obstacle and bacterial proliferation in the bile. The increase in intraductal pressure, in turn, leads to bacterial translocation, resulting in bacteremia [2, 4-6].
The clinical diagnosis of acute cholangitis is based on Charcot's triad: fever, pain and jaundice, in which the most common symptoms are fever and abdominal pain, in up to 80% of patients [4,7]. There are several etiologies of acute cholangitis. However, recurrent episodes of cholangitis should prompt the search for a triggering factor, such as biliary obstruction [4,7].
Infections are the second most frequent cause of mortality in end-stage renal disease patients. Bacteremias caused by gram-negative agents are less frequent. However, its prevalence has been increasing. The most frequent focus of infection is vascular access for hemodialysis and limb wounds. Mortality from bacteremia caused by negative agents in patients undergoing a regular hemodialysis program is greater than 25% [8].
Clinical Case
We report the case of a 69-year-old man in a regular hemodialysis program for autosomal dominant polycystic kidney disease (ADPKD) with no residual diuresis admitted to the Nephrology service for the 5th time in one year period with fever (axillary temperature: 39 °C), with onset during hemodialysis session, and altered state of consciousness. The patient was on regular HD program for 3 years with a functioning left umerocephalic arteriovenous native fistula and suffered from hypertension, obesity (BMI 34Kg/m2) and preserved ejection fraction heart failure. The patient was on losartan 100mg once daily, bisoprolol 2.5mg once daily, 100mg acetylsalicylic acid once daily and omeprazol 20mg once daily.
The patient had a relevant history of 4 previous hospitalizations for fever of unknown etiology, with no further sign or symptom, secondary to broad-spectrum beta-lactamase producer E.Coli, only sensitive to carbapenems and colistin. In previous hospitalizations pulmonary, kidney and liver cysts infection were excluded through computer tomography. Native AV fistula infection had also been excluded in every previous hospitalization through physical examination and ecographic evaluation. The patient had colonic diverticulosis as part of his ADPKD and had performed colonoscopy twice on previous hospitalizations for exclusion of colonic diverticulitis. As no evident source of infection was found, the patient had been treated with an empiric course of meropenem (load dose of 1gr, followed by 500 mg per day) for 10 to 14 days on the previous hospitalizations. On every previous hospitalization the patient showed marked improvement after directed antibiotic therapy was initiated with resolution of both fever and inflammatory markers on blood work.
On his 5th hospitalization the patient started complaining of abdominal pain, of sudden onset, tightening type and moderate intensity 6/10 on the first 24 hours after hospitalization. In association, he described chills, feeling of postprandial fullness and nausea. The patient denied fatigue, vomiting or other changes in intestinal transit, such as diarrhea, recent trips, contact with animals, consumption of untreated water or pasteurized products.
On physical examination, the patient was hypotensive, tachycardic and presented a globose abdomen, painful on deep palpation of the right hypochondrium and epigastrium, with slight defense and absent Murphy's sign. Full skin, no wounds and no jaundice. On blood work he had leukocytosis (16 x 10³ /μL) with neutrophilia (11.51 x 10³ /μL), marked elevation of C-reactive protein (414.5 mg/L). Hepatic laboratory tests showed a slight rise of aspartate transaminase (52U/L), alanine transaminase (71 U/L), Alkaline phosphatase (300 U/L), gamma-Glutamyl Transferase (255 U/L) and mild hyperbilirubinemia (2.1 mg/dL) mostly due to direct bilirubin (1.3 mg/dL).
Abdominal tomography at this time showed dilatation and ectasia of the main biliary tract (MBT). Blood cultures (BC) were positive again for broad-spectrum beta-lactamase producer E.coli with the same pattern of antibiotic resistances as previously reported. It was also found that the tumor marker CA19.9 and chromogranin A were altered, the latter being markedly elevated.
The case was discussed with Gastroenterology and General Surgery departments. We chose to perform an upper digestive endoscopic examination, in which a formation that prolapsed from the lumen into the MBT, which corresponded to Vater's papilla (Figure 1), was seen. This was followed by duodenoscopy, in which progression to DII was possible, where initially the papilla could not be seen by mobilization into the insides of an apparent ampullary IDD (Figure 2A). Using a biopsy forceps, it was possible to pull and exteriorize the papilla, which had increased dimensions (approximately 15mm) and villous morphology, findings suggestive of ampuloma (Figure 2B and 2C). The papilla was biopsied and the anatomopathological report revealed a tubular adenoma with low-grade dysplasia.
The patient completed antibiotic therapy cycle with meropenem, rifaximin and ursodeoxycholic acid for 3 weeks with complete resolution of all complaints. Repeated blood cultures were negative. After discharge, he was referred to hepatobiliary team consultation.
In this case, endoscopic resection would have been ideal, and was the first approach, however on the second duodenoscopy (2 months after initial diagnosis) the ampulloma had grown and could not be mobilized from the main biliary duct. The surgical team proposed a transduodenal surgical ampullectomy, even though the dimensions of the tumour and expected scarring due to recurrent inflammation, would make it technically difficult for the biliary and pancreatic duct reconstruction, and the risk of intra-operative complications would be very high. Meanwhile, the patient-maintained episodes of bacteremia with sepsis every 2 months and his clinical status had worsened and he had been losing weight. A multidisciplinary clinical decision-making consultation with the patient was held 8 months after ampulloma diagnosis was held. The anesthesiology and surgical team considered the patient very polymorbid (end stage renal disease, obesity, recent weight loss, heart failure with preserved ejection fraction) for such major surgery, especially as the biliary tract would be colonized with a multiresistant bacteria which would leave the patient at an extreme risk for intra operative and post operative complications. The patient refused the surgery and opted for a palliative option. Gastroenterology department performed an endoscopic sphincterotomy and biliary plastic stent placement with good drainage after the procedure (Figure 3).
Despite the success of the treatment, the patient returned to the hospital 4 more times with sepsis and without signs of biliary obstruction. The same pathogenic bacteria was isolated on blood cultures. Patient eventually died from septic shock 9 months after stent placement. After his death, blood cultures revealed E. Coli resistant to carbapenems, which explains why on his last hospitalization the patient did not respond to antibiotic therapy and progressed to septic shock.
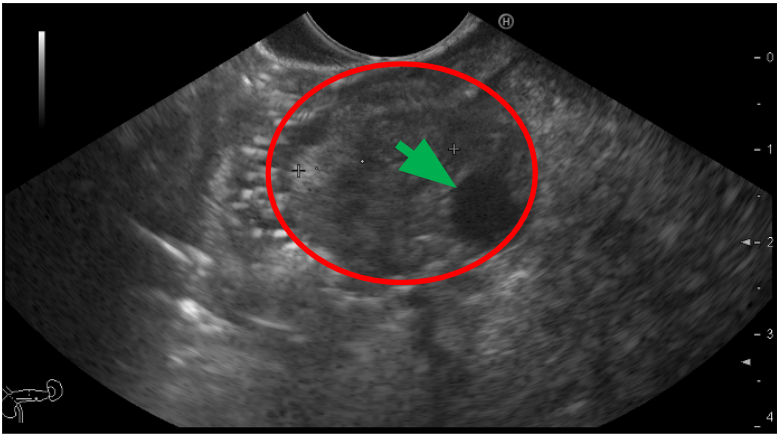
Figure 1: Upper digestive endoscopy image. The ampuloma is represented in red. The common bile duct is highlighted with a green arrow.

Figure 2: Duodenoscopy images in DII. A) Vater's papilla inside IDD; B) Papilla traction with forceps; C) Externalized papilla with villous appearance.
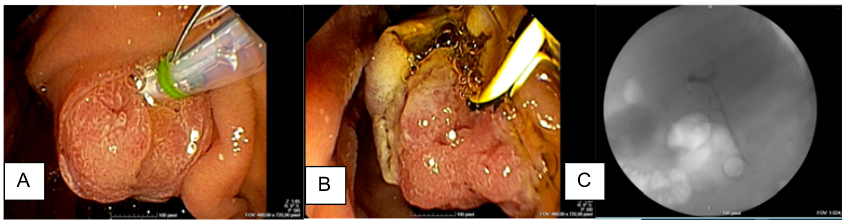
Figure 3. Duodenoscopy images of palliative stent placement. A) Villous ampuloma; B) Sphincterectomy; C) Plastic stent placement on fluoroscopy image (Double pigtail 10Frx5 cm stent)
Discussion
We presented a case of a rare cause of recurrent sepsis in a hemodialysis patient with ADPKD and all the difficulties associated with diagnosis and definitive treatment due to the comorbidities of this type of patient.
Sepsis in patients with ADPKD is mainly caused by kidney or infected liver cysts infection. In this case, patient's complaints and complementary diagnostic tests only pointed to biliary tract origin on the 5th hospitalization which delayed the etiological diagnosis and certainly impacted negatively on the patient prognosis.
Vater's ampoule tumors are infrequent and in 2% of cases are malignant. The most common are adenomas (70% are villous adenomas) and adenocarcinomas. They appear predominantly in the 6th decade of life, without gender predominance. Clinical manifestations usually result from biliary tract obstruction and may include constitutional symptoms: increased liver enzymes, obstructive jaundice and, less frequently, episodes of acute cholangitis [9,10]. In cases of recurrent cholangitis, morphological changes in the biliary tract and other causes of biliary obstruction must be excluded [4,9,11,12].
Biliary obstructions, in most cases, are accompanied by upstream ductal dilatation, ultrasound being an imaging exam with increased sensitivity in detecting intra- or extrahepatic biliary dilatation. However, endoscopic echography or echo-endoscopy, with endoscopic retrograde cholangiopancreatography, demonstrates greater sensitivity and specificity and allows the collection of samples such as tissue biopsy [9].
Serum measurement of tumor markers also contributes to greater suspition of neoplasia. It is known that CA 19.9 is expressed by the pancreatobiliary epithelium and may also be increased in episodes of cholestasis. Chromogranin A has high sensitivity and specificity for neuroendocrine tumors, the gastrointestinal location being the most common, and Vater's ampoule one of the reported locations [12].
Our patient had an intraluminal duodenal diverticulum, which made diagnosis even harder. Association of autosomal dominant polycystic kidney disease and colonic diverticulosis is well established, however whether there is a genetic correlation between between ampullary lesions, IDD and ADPKD is uncertain [13]. On a series of 8 cases of duodenal diverticula arising in patients with ADPKD published in 2006, the majority of patients (6) developed biliary complications, 5 of which had duodenal diverticula in the second part of the duodenum near the ampulla of Vater, but only one developed cholangitis with bacterial overgrowth. On the referred series 3 patients were end-stage renal disease (3 of them on haemodialysis and one after renal transplantation), so the authors suggested an increased occurrence of duodenal diverticular disease in ADPKD patients with ESRD [14].
On the reported case, the patient not only had the presence of an intra duodenal diverticulum, but also an ampulloma hidden inside. To the best of our knowledge, this is the first such presentation in this population worldwide. Acute cholangitis as a form of presentation only occurred once, on the hospitalization that actually led to a final diagnosis. The most frequent clinical presentation was sepsis without cholestasis, suggesting a predominance of bacterial overgrowth and translocation rather than biliary obstruction.
The first line of treatment of benign biliary tract obstruction is, in most cases, medical and endoscopic, with endoscopic dilation, with or without stent placement [9]. E. Coli and Klebsiella species are the most frequent pathogenic agents in biliary tract infections4 therefore antibiotic therapy usually combines a 3rd generation cephalosporin associated with an anaerobic agent [4,5]. As reported in our case, the E. coli isolated in the blood cultures was a multidrug resistant bacterium which forced us to use meropenem from the beginning.
Villous-looking ampuloma is considered a pre-malignant lesion, evolving to adenocarcinoma in 25-40% of cases, and should be subsequently addressed with endoscopic resection if feasible (mucosal involvement only) or surgical treatment by transduodenal surgical ampulectiomy or radical pancreaticoduodenectomy, depending on the patient physical condition, intraoperative or preoperative biopsy or suspicion for metastatic lymph nodes [1,10].
Timely surgical treatment would have been the only definite treatment for our patient. However, recurrent bacterial overgrowth and recurrent hospitalizations rapidly deteriorated our patient and greatly increased his risks for a major surgery. Final outcome was foreseeable as antibiotic resistance is a known risk of repeated courses of antibiotic therapy and this could make for a whole new discussion.
In conclusion, the authors would like to remark that an ampuloma can infrequently cause bacterial overgrowth causing sepsis without necessarily developing cholestasis, requiring a high degree of suspicion for diagnosis. Timely diagnosis is essential as even benign tumors can grow and become unsuitable for endoscopic resection which is the safest modality of treatment. Patients on a regular hemodialysis program are at higher risk of surgical complications and postoperative mortality when compared to the population with normal renal function, and the benefit-risk must be weighed on a case-by-case basis.
Ethics approval and consent to participate
Ethical committee approval was not needed as article case doesn’t include research. Written consent from the patient was obtained for all procedures.
Consent for Publication: Written informed consent for publication of their clinical details and images was obtained from the patient.
Competing Interests: The authors declare that they have no competing interests.
References:
- Aceñero M, Useros J, Díez-Valladares L, Ortega-Medina L, Aguirre E, Esteban S, et al. Factores pronósticos en el adenocarcinoma de ampola duodenal. Rev Esp Patol, 2018; 51: 210-215. doi: 10.1016/j.patol.2018.03.001.
- Lima J, Rebelo M, Castellano A, Mota J, Ruivo C, Dias P, et al. Gastroenterite eosinofílica: divertículo duodenal intraluminal como factor predisponente? GE J Port Gastrenterol, 2012; 19: 146-150. doi: 10.1016/j.jpg.2012.04.002.
- Oukachbi N, Brouzes S. Management of complicated duodenal diverticula. J Visc Surg, 2013; 150: 173-179. doi: 10.1016/j.jviscsurg.2013.04.006.
- Sokal A, Sauvanet A, Fantin B, Lastours V. Acute cholangitis: Diagnosis and management. J Visc Surg. 2019; 156: 515-525. doi: 1016/j.jviscsurg.2019.05.007.
- Uno S, Hase R, Kobayashi M, Shiratori T, Nakaji S, Hirata N, et al. Short-course antimicrobial treatment for acute cholangitis with gram-negative bacillary bacteremia. J Infect Dis. 2017; 55: 81-85. doi: 10.1016/j.ijid.2016.12.018.
- O´Connor M, Summer H, Schwartz M. The clinical and pathologic correlations in mechanical biliary obstruction and acute cholangitis. Ann Surg. 1982; 195: 419-423. doi: 1097/00000658-198204000-00007.
- Wah D, Christophi C, Muralidharan V. Acute cholangitis: current concepts. ANZ J Surg, 2017; 87: 554-559. doi: 10.1111/ans.13981
- Murray E, Marek A, Thomson P, Coia J. Gram-negative bacteraemia in haemodialysis. Nephrol Dial Transplant, 2015; 30: 1202-1208. doi: 10.1093/ndt/gfv205.
- Altman A, Zangan M. Benign biliary strictures. Semin Intervent Radiol, 2016; 33: 297–306. doi: 10.1055/s-0036-1592325.
- Guidirim G, Misin I, Istrate V, Cazacu S. Endoscopic papillectomy into the treatment of neoplastic lesions of Vater papilla. Curr Health Sci J, 2009; 35: 92-97.
- Hasegawa E, Sawa N, Hoshino J, Suwabe T, Hayami N, Yamanouchi M, et al. Recurrent cholangitis in a patient with Autosomal Dominant Polycystic Kidney Disease (ADPKD) and Caroli’s Disease. Intern Med, 2016; 55: 3009-3012. doi: 10.2169/internalmedicine.55.6818.
- Malaguarnera G, Giodarno M, Paladina I, Rando A, Ucello M, Basile F, et al. Markers of bile duct tumors. World J Gastrointest Oncol, 2011; 15: 49-59. doi: 10.4251/wjgo.v3.i4.49
- Scheff RT, Zuckerman G, Harter H, Delmez J, Koehler R. Diverticular disease in patients with chronic renal failure due to polycystic kidney disease. Ann Intern Med. 1980; 92(2, pt 1): 202–204. doi: 10.7326/0003-4819-92-2-202.
- Kumar S, Adeva M, King BF, Kamath PS, Torres VE. Duodenal diverticulosis in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant, 2006; 21(12): 3576–3578. doi: 10.1093/ndt/gfl405.

