Hepatic Injury Induced by Moringa Oleifera with Rechallenge
Vinicius Nunes* and Catarina Secundino, Sarah de Souza Lira Gameleira, Raymundo Paraná,Maria Isabel Schinoni
Gastroenterology Department, Hospital Universitário Professor Edgard Santos, Brazil
IDOR Researcher, Brazil
Medical Student, Universidade Federal da Bahia, Brazil
Received Date: 07/02/2022; Published Date: 17/02/2022
*Corresponding author: Vinicius Santos Nunes, MD Email: snunesvinicius02@gmail.com *
Correspondence: Gastroenterology Department, Ambulatório Professor Francisco Magalhães Neto, R. Padre Feijó, No. 240, Canela, 40110-170, Salvador, Bahia, Brazil. Tel +557132838380
*Both authors contributed equally to this paper
Abstract
Herb Induced Liver Injury (HILI) is a condition in which there is aggression to the liver tissue after exposure to substances present in many different medications, dietary supplements, herbs and xenobiotics. Diagnosing HILI is challenging given that many other causes must be previously excluded, but it should always be considered since it's one of the most frequent causes of acute liver failure in the West. We report a case of a 60-year-old female who used Moringa oleifera and presented with fatigue and elevated liver enzymes, which resolved after she suspended its consumption.
Keywords: Case report; DILI; HILI; Moringa oleifera; Drumstick tree
Introduction
Herb Induced Liver Injury (HILI) has become an increasingly frequent cause of damage to liver tissue and it’s one of the most frequent causes of acute liver failure in Western countries [1]. It’s defined as an elevation of ALT (alanine aminotransferase) in at least 5 (five) times its upper limit of normality (ULN) or an elevation of ALP (alkaline phosphatase) in at least 2 (two) times its ULN [2]. In case the patient presents with associated symptoms or bilirubin elevation, ALT values above 3 (three) times its ULN must be considered [2].
In order to diagnose hepatotoxicity, there must be compatible chronology between drug use and liver injury (within 6 months of drug use) [1,3] and alternative causes must be excluded. Clinical manifestations of the condition may vary from nothing to abdominal discomfort, rash, fever, jaundice and/or liver failure [1,2]. There’s a helpful tool to diagnosing HILI called “Roussel Uclaf Causality Assessment Method (RUCAM)”, which consists of calculating the R-value (to determine the injury pattern, which can be cholestatic, hepatocellular or mixed) and going through a checklist of case-related clinical features [4,5].
Normalization in biochemical patterns after the suspected compound is suspended (“dechallenge”), recurrence of injury after re-administration of the compound (“rechallenge”) and the likelihood that the compound is hepatotoxic (previously known cases) also help in stablishing the diagnosis [1].
Herbal and Dietary Supplements (HDS) is a term that embraces an incredible variety of natural and artificial compounds, some of which cause hepatotoxicity. Moringa oleifera, also known as “drumstick tree”, is a traditional herb originally from South Asia that has been spread across the planet and used for many different purposes in over thousands of years. It has been recently getting a lot of attention from the scientific community given its health and nutritional benefits [6], which has been used as a strong argument to explore Moringa as an important food source in developing countries and food fortification [7,8]. Some of its health benefits include (but are not limited to): analgesic, antioxidant, antipyretic and anti-inflammatory activities [6,8].
Many authors have already claimed that M. oleifera also has hepatoprotective effects in decreasing serum levels of ALT, AST and ALP after some studies in mice have shown positive results [6], but most of them can’t offer useful data since they have not tested Moringa in human beings [7]. From our research, no hepatotoxic effects of the plant had been reported until now.
Case Presentation
A 60-year-old female had been previously diagnosed with hypothyroidism (under treatment with Levothyroxine) and dyslipidemia and had a past medical history of melanoma and gastroesophageal reflux disease. She presented with an episode of mild acute hepatitis with no compromise to liver function in June/2019, which was shown by a slight elevation of ALT and AST (aspartate aminotransferase) as seen in Table 1. After exclusion of alternative etiologies (Table 2), hepatotoxicity was suspected due to the patient’s recent use of Ezetimibe and Moringa oleifera in capsules one month prior to the altered laboratory exams.
Both medications were suspended on June 16th, 2019 and liver enzymes reached near normal values two months later (08/17/2019 – Table 1). Against medical recommendation, the patient restarted consumption of Moringa oleifera (no longer associated with Ezetimibe) on August 17th, 2019, and presented to the clinic with fatigue and altered laboratory exams (09/12/2019 - Table 1) one month later.
The patient was submitted to an abdomen ultrasonography, which showed no structural abnormalities on September 16th, 2019, and a cholangioresonance on October 24th, 2019, which showed a renal cyst and aortic atheromatous disease. She was oriented to suspend consumption of Moringa oleifera on September 13th, 2019. From then on, there was a steady clinical recovery and improvement in biochemical patterns (10/14/2019 – Table 1). Complete normalization of liver enzymes was noted on November 9th, 2019 (Table 1).
This case’s diagnosis was validated by an international group dedicated to studying hepatotoxicity (International Latin-American and Spanish Cohort for Drug Induced Liver Injury) [10]. This case report was prepared according to the CARE guidelines [11].
Table 1: Biochemical Data.
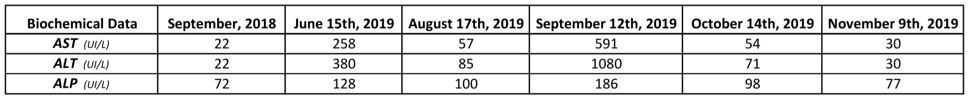
Reference values: AST (15-46 UI/L); ALT (11-69 UI/L); ALP (38-126 UI/L). The patient’s R-value is 8.54. Data from September/2018 serves as a reference point for the patient, but it’s not useful in this calculation.
Table 2: Laboratory Tests for Viral and Autoimmune Antibodies.
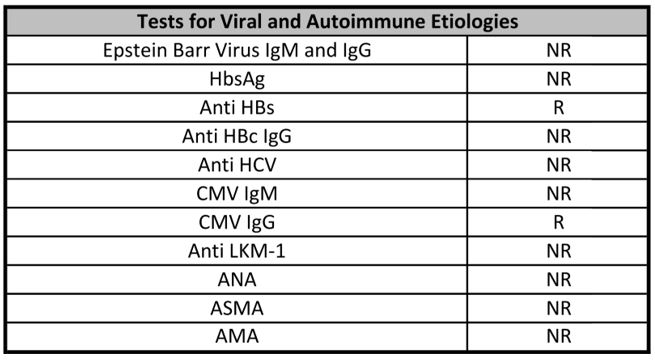
HbsAg: Hepatitis B virus surface antigen; Anti HBs: Hepatitis B surface antibodies; Anti HBc: Hepatitis B core antibodies; Anti HCV: Hepatitis C virus antibody; CMV: cytomegalovirus antibodies; Anti LKM-1: Liver kidney microsome type 1 antibodies; ANA: antinuclear antibodies; ASMA: Anti Smooth Muscle antibodies; AMA: antimitochondrial antibodies; NR: Not Reactive; R: Reactive. Although the patient had positive results for Anti HBs and CMV IgG, we concluded that hepatotoxicity fit her case better considering chronology, dechallenge, rechalllenge and her clinical presentation.
Graphic 1: Variation of Biochemical Data.

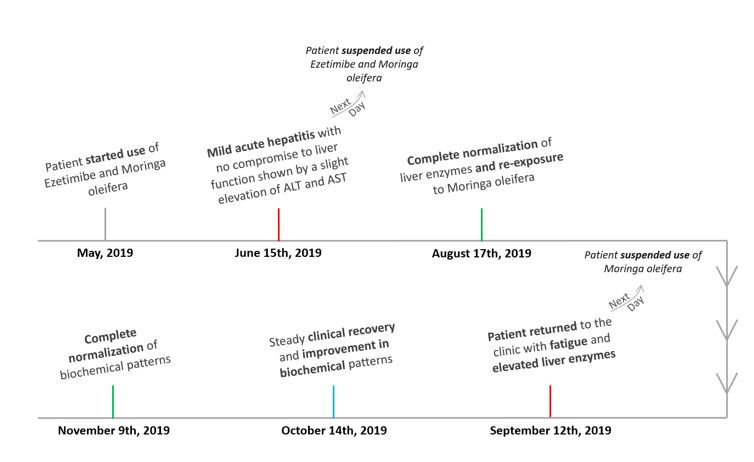
Figure 1: Timeline.
Discussion
Herb Induced Liver Injury has been a growing cause of liver disease worldwide in the past few years, especially in developing regions (such as in Latin America and Asia) [3,4]. In many of these countries, an aggravating factor for the increasingly high number of HILI cases is the lack of strict regulations for the use of many of these herbs as the ones that already exist for drugs [4]. Furthermore, given how difficult diagnosing HILI can be, a lot of cases are not notified, which makes it an underreported condition [2,4].
This case report presents a typical HILI pattern with rechallenge to the drug. Liver inflammation is usually quicker on a rechallenge, so it imposes a higher risk when compared to the first exposure and it’s usually heavily discouraged (unless it’s a critical treatment) [3].
Our patient presented multiple clues that indicated hepatotoxicity due to M. oleifera, such as compatible chronology, dechallenge, rechallenge and exclusion of alternative causes. Despite a low likelihood (as there have been reports that this herb is hepatoprotective) [6], Moringa could be damaging to the liver considering that alkaloids (important class of hepatotoxic agents [2]) have already been detected in its composition [6]. Furthermore, RUCAM was favorable towards our diagnosis: her R-value was 8.54, which is compatible with hepatocellular hepatotoxicity, and her RUCAM score was 6 (hepatotoxicity due to Moringa is “probable”) [5].
A problem we faced in this case is something quite frequent when dealing with HILI: the uncertainty when it comes to dosage of the suspected substance and the accurate composition of the mixtures [2,4]. Therefore, it’s often hard to pinpoint one single agent responsible for the inflammatory reaction in the liver, which also makes the diagnosis harder. In our case, we have an unknown daily dosage of Moringa oleifera being self-administered by the patient following no schedule.
Conclusion
Even though Moringa is widely used as a dietary complement, its composition can be harmful, especially considering that its consumption doesn’t always address efficacy, dosage, quality and safety. Thus, case reports (such as this) are important to help identify herbs that are commonly considered to be beneficial, but that can also be toxic to our health.
Acknowledgements
Our group highlights the support received from CNPq, FAPESP, Maria Emilia Foundation (FME) and IDOR to support the research line in neglected liver diseases (HILI, DILI, Hepatitis D and Obliterative Portal Venopathy).
Mario Jorge Souza Cruz – Bed 04], Ana Maria de Jesus went to the infirmary, Joelma – walks with the help of 2 people, Senilda did not walk because she was recently thrombolysed.
References
- Hoofnagle JH, Björnsson ES. Drug-Induced Liver Injury - Types and Phenotypes. Longo DL, editor. New England Journal of Medicine, 2019; 381(3): 264–273.
- Nunes V, Mendez‐Sanchez N. Impact of Herbal and Dietary Supplements Causing Drug‐Induced Liver Injury in Latin America. Clinical Liver Disease, 2020; 16(3): 83–86.
- Hunt CM, Papay JI, Stanulovic V, Regev A. Drug rechallenge following drug-induced liver injury. Hepatology, 2017; 66(2): 646–654.
- Bessone F, Hernandez N, Tagle M, Arrese M, Parana R, Méndez-Sánchez N, et al. Drug-induced liver injury: A management position paper from the Latin American Association for Study of the liver. Annals of Hepatology, 2021; 24: 100321.
- Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. Nih.gov. National Institute of Diabetes and Digestive and Kidney Diseases; 2019.
- Kumar S, Bhattacharya A, Tiwari P, Sahu P. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. Journal of Pharmacy and Bioallied Sciences, 2018; 10(4): 181.
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. International Journal of Molecular Sciences. 2016; 17(12): 2141.
- Bessone F, Hernandez N, Mendizabal M, Sanchez A, Paraná R, Arrese M, et al. When the Creation of a Consortium Provides Useful Answers: Experience of The Latin American DILI Network (LATINDILIN). Clinical Liver Disease, 2019; 13(2): 51–57.
- Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. Journal of Clinical Epidemiology. 2017; 89: 218-235.

