Acquired Thrombotic Purpura Successfully Treated with Caplacizumab without Plasma Transfusion in a Jehovah’s Witness Patient
Ruchi Desai MD, Ankita Tandon DO, Michael Jaglal MD*
P1University of South Florida Morsani College of Medicine, Tampa, FL, USA
2H. Lee Moffitt Cancer Center, Tampa FL, USA
Received Date: 07/06/2021; Published Date: 23/06/2021
*Corresponding author: Ricardo Mesquita Camelo, MD, University of South Florida, Morsani College of Medicine, Department of Internal Medicine and Division of Hematology, H. Lee Moffitt Cancer Center, 1209 USF Magnolia Drive, GME Office, Tampa, FL 33612. Email: Michael.jaglal@moffitt.org
Abstract
Acquired Thrombotic Thrombocytopenic Purpura (TTP) is a hematologic emergency characterized by auto-antibody mediated inhibition or clearance of ADAMTS13, a von Willebrand Factor (vWF) cleaving metalloprotease, thrombocytopenia, microvascular thrombosis, and hemolytic anemia. The primary treatment of TTP is therapeutic plasma exchange with human plasma transfusion, a procedure known as PLEX. Caplacizumab, an immunoglobulin fragment that inhibits vWF and platelet interaction, has been shown to improve outcomes when used in conjunction with PLEX and immunosuppression. For Jehovah’s Witness patients who do not accept plasma transfusion, optimal treatment is uncertain. We present a case of TTP successfully treated with delayed caplacizumab without plasma transfusion. Our patient’s experience provides evidence that caplacizumab with immunosuppression even in the absence of PLEX may be a feasible alternative for treatment of TTP in patients who are unwilling or unable to accept plasma transfusion. Our case also provides evidence for use of caplacizumab as a step-up therapy in patients who fail initial treatment with therapeutic plasma exchange.
Introduction
Acquired Thrombotic Thrombocytopenic Purpura (TTP) is a Thrombotic Microangiopathy (TMA) characterized by auto-antibody mediated inhibition of von Willebrand Factor (vWF) cleaving metalloprotease ADAMTS13. Lack of ADAMTS13 activity results in unrestrained platelet and vWF aggregation, platelet consumption, hemolytic anemia, and microvascular thrombosis [1]. The consequential tissue ischemia and infarction can result in multisystem organ failure, neurologic damage, and death. If untreated, mortality rate from TTP is >90% [2].
Therapeutic plasma exchange with human plasma transfusion, a procedure known as PLEX, is the principal treatment of TTP since clinical trials demonstrated its effectiveness [3]. Plasma exchange rapidly removes auto-antibodies and vWF multimers and plasma transfusion replenishes ADAMTS13 activity, restoring coagulation homeostasis [3,4]. Definitive control of TTP is accomplished with immunosuppression over the course of weeks [1,5]. Caplacizumab (Cablivi®, Ablynx, N.V. Belgium), a bi-valent immunoglobulin fragment that inhibits platelet GP Ib-IX-V receptor and vWF interaction, has recently been shown to be a beneficial adjunctive medication to PLEX and immunosuppression for treatment of TTP. In clinical trial, patients treated with caplacizumab had more rapid platelet recovery, lower incidence of TTP related death or thrombosis, lower recurrence rate of TTP, underwent fewer PLEX treatments, and had shorter hospital stay [6]. In addition, patients had a 38% reduction in time to normalization of markers of end organ damage including LDH, serum creatinine, and troponin [7].
Treatment of TTP in Jehovah’s Witness followers poses a unique challenge. The Jehovah’s Witness religious doctrine rejects transfusion of human whole blood and its products, precluding the use of PLEX [8]. Infusion of albumin or purified proteins is not strictly prohibited, and some patients may accept them. Here, we report successful management of a Jehovah’s Witness patient treated with caplacizumab without PLEX for treatment of TTP.
Case Presentation
A 25 years old woman, who is a Jehovah’s Witness, presented with complaints of dark urine. She was otherwise asymptomatic without fevers, dysuria, abdominal pain, nausea, vomiting, confusion, headaches, weakness, or fatigue. At presentation she was normotensive, afebrile, and pulse rate was 90 bpm. Her physical examination showed petechiae but was unremarkable without evidence of pallor, jaundice, scleral icterus, hepatosplenomegaly, bruising, or costovertebral angle tenderness. She had medical history of TTP diagnosed three years previously that had been treated successfully.
Urine analysis revealed hematuria without erythrocytes, leukocytes, or casts. Urine culture did not have evidence of infection. Laboratory studies showed white blood cell count 9.13 x 103/µL with normal differential, hemoglobin 13.8 mg/dL, platelet count 41 x 103/µL, creatinine 1.5 mg/dL, lactate dehydrogenase 1021 U/L (>4 times upper limit of normal), and undetectable haptoglobin (<8 mg/dL). Four days prior to presentation, platelet count was 230 x 103/µL and creatinine 0.7 mg/dL. Total and conjugated bilirubin, alanine transferase, aspartate aminotransferase was within normal limits. Peripheral blood smear revealed schistocytes and absent platelets. Activated partial thromboplastin time (aPTT), prothrombin time (PT), and fibrinogen were within normal limits. Screening tests for HIV and Hepatitis C were negative. ADAMTS13 activity was undetectable (<3%) with functional inhibitor >32 Bethesda Units (BU), confirming relapsed TTP.
PLEX was recommended, but the patient declined plasma transfusion. She did accept cryoprecipitate and albumin. Daily plasma exchange with albumin replacement (1:1 plasma volume) was commenced and she completed five treatments. Cryoprecipitate was transfused twice to keep fibrinogen >100. In addition, erythropoietin and folic acid were started for bone marrow stimulation. Immunosuppression was administered consisting of methylprednisone 1000mg daily (Days 1-3), rituximab 375 mg/m2 (Day 1), and vincristine 2mg (Day 3). Methylprednisone was decreased to prednisone 1mg/kg starting Day 4 and was subsequently tapered over the course of eight weeks. Rituximab was given weekly for four cycles, and vincristine was given weekly for four cycles. Her creatinine normalized to 0.8 mg/dL but she continued to have progressive thrombocytopenia with platelet count 7 x 103/µL and anemia with Hgb 7.7 mg/dL on Day 4. Her LDH trended downwards but remained slightly elevated and she continued to have undetectable haptoglobin, indicative of ongoing hemolysis.
Caplacizumab 10mg daily was added on Day 5 and continued for 30 days. The first dose was given intravenously, and subsequent doses were given subcutaneously, per manufacturer’s recommendations. Within 24 hours of first caplacizumab dose, platelet count increased to 21 x 103/µL, haptoglobin normalized to 43 mg/dL, and LDH normalized (177 U/L) indicating improvement in hemolysis. Platelet count normalized by Day 7 and hemoglobin began improving by Day 10, though did not normalize until completion of immunosuppression. ADAMTS13 activity remained low (<10%) with functional inhibitor titer present (although reduced 0.5 BU) on Day 6. The patient was discharged from the hospital on Day 10.
She was followed closely in the outpatient setting for two months. ADAMTS13 activity was restored and inhibitor suppressed by Day 35. On Day 59 follow up, ADAMTS 13 remained normal without inhibitor, consistent with remission. She experienced mild epistaxis while taking caplacizumab which was treated with oral tranexamic acid. Epistaxis coordinated with low vWF activity (10%) with normal vWF antigen (84%) and improved after discontinuation of drug (vWF activity 130% on Day 59) (Figure 1).
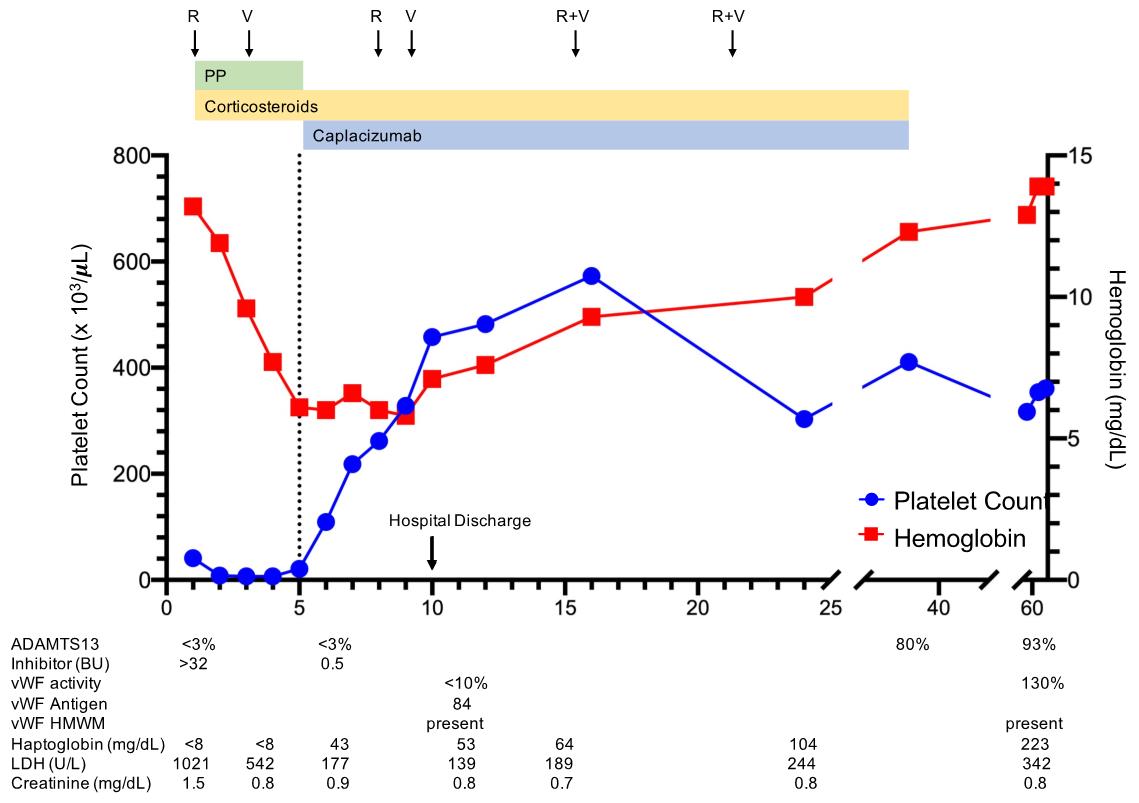
Figure 1: Treatment of Relapsed (TTP) in a Jehovah’s Witness Patient. The clinical course of a 25 year old patient with relapsed TTP treated without plasma transfusion is shown. The patient was treated with an initial combination of plasma exchange with albumin replacement (PP), caplacizumab, and immunosuppression with corticosteroids, rituximab (R), and vincristine (V). She achieved remission with recovery of ADAMTS13 by Day 35.
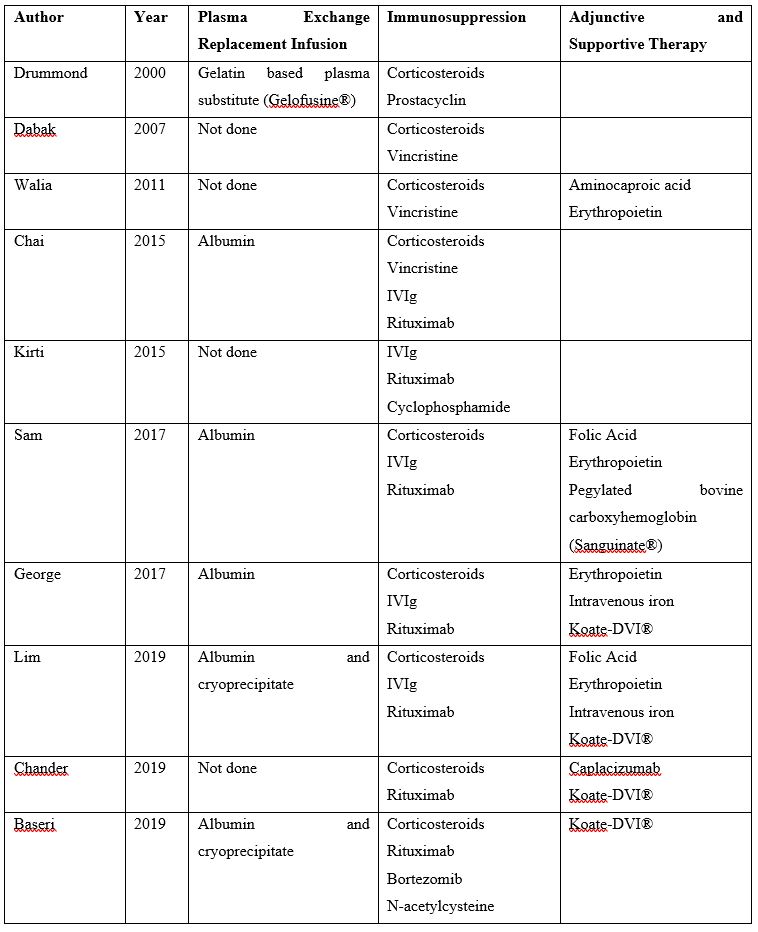
Supplementary Table 1: Previous case reports of TTP in Jehovah’s Witness patients managed without therapeutic plasma exchange with plasma infusion (PLEX)
Discussion
There are no guidelines for the management of TTP without plasma transfusion and the optimal strategy is not known. Ten cases of TTP in Jehovah’s Witness patients managed successfully without plasma transfusion are described in the literature (9-18) (Supplemental Table 1). However, we suspect that there are many cases with poor outcomes that are not reported, as the mortality for TTP prior to PLEX was high.
In all ten cases, long term control was achieved with immunosuppression utilizing a combination of corticosteroids, rituximab, and/or chemotherapy though emergent therapy varied [9-18]. Six cases report use of therapeutic plasma exchange with alternative protein replacement [9,11,13,15,16,18]. Recently, Chander et al. were the first to describe effective TTP treatment in a patient with caplacizumab for 30 days and immunosuppression without PLEX or plasma infusion. Their patient had clinical improvement within 24 hours of caplacizumab administration and normalization of platelet count within three days. On follow up several weeks later, the patient remained in remission [17].
To our knowledge, our patient’s experience is only the second case to report use of caplacizumab without PLEX for successful treatment of TTP. In our patient’s experience, treatment with caplacizumab on Day 5 without therapeutic plasma exchange or plasma transfusion resulted in clinical improvement of TMA with normalization of platelet count and markers of end organ damage (LDH) within three days of administration. These results are similar to those noted by Chander et al. and in the HERCULES study, in which the 75th percentile for platelet normalization was 2.95 days (95% CI 2.85-3.91) and median time to normalization of organ damage markers was 2.86 days (95% CI 1.93-3.86) with PLEX and caplacizumab [6]. Our patient’s ADAMTS13 activity remained undetectable after starting caplacizumab, despite improvement in thrombocytopenia, hemolysis, and end organ damage. At the same time, vWF antigen with high molecular weight multimers were still detectable though vWF activity was low. These data suggest that caplacizumab, independent of ADAMTS13 activity, was effective in preventing platelet and vWF adhesion and thus microvascular thrombosis.
This experience also demonstrates that caplacizumab alone is not sufficient treatment for TTP, but rather a supportive measure until immunosuppression can take full effect. Rituximab has been studied in front line therapy and is used by many physicians to treat acute TTP. It’s benefit has been reported in several cases of Jehovah’s witness patients and in two prospective cohort studies and one retrospective registry study in combination with PLEX and corticosteroids [5,19]. In these studies, remission from TTP has been reported to occur within 14-21 days. In addition, reduced risk for relapse is noted with rituximab use [5,19]. Vincristine, a microtubule inhibitor without megakaryocyte activity, has been noted to have variable efficacy in observational series in both the relapsed and acute setting (20, 21), with combination vincristine and corticosteroids reporting 50-100% response rates. Vincristine use is also reported with success in Jehovah’s Witness patients with TTP [9,10,12]. In our patient’s case, corticosteroids, rituximab, and vincristine were all safely administered concurrently and resulted in eradication of the ADAMTS13 functional inhibitor with restoration of ADAMTS13 activity by Day 30. It is possible that the patient achieved remission prior to Day 30 as she clinically improved, but we did not check data prior to Day 30 as it would not alter our management plan.
Despite favorable outcome, our patient’s case highlights challenges with caplacizumab treatment. The major barrier to caplacizumab use is cost and lack of widespread availability of the drug. At the time of this publication, caplacizumab cost is around US$8000 per dose [22]. With treatment regimens of 30 days and relative rarity of TTP, the drug may not be stocked in many hospitals and may require insurance approval prior to use, which could delay treatment. For patients who present with aggressive disease with end organ damage, high LDH, and high functional inhibitor titer, waiting for drug procurement to start therapy may not be feasible. There are case reports of caplacizumab used as step-up therapy based on ADAMTS13 activity for those failing standard treatment, but no “real-world” cohort or clinical trial data currently exists regarding this approach or delayed treatment [23].
In our patient’s case, caplacizumab was not available for emergent administration and took five days to procure. She presented with renal failure, high LDH, as well as high inhibitor titer suggesting aggressive disease. Studies show that one volume of plasmapheresis removes approximately 45% of circulatory IgG and three to five days of plasmapheresis can remove up to 90% of circulating IgG [4]. We employed therapeutic plasma exchange with albumin replacement in addition to immunosuppression to reduce vWF multimers and circulating antibodies. With this approach, functional inhibitor titer decreased, but did not resolve and ADAMTS13 activity remained low (<10%). This phenomenon likely resulted from a combination of removal of autoantibody without replenishment of exogenous ADAMTS13 from lack of plasma transfusion in addition to ongoing inhibition of endogenous ADAMTS13 by a strong inhibitor. Markers of hemolysis did not change dramatically in our patient’s case by the last day of plasma exchange, though reduced LDH and improvement in renal function was noted. We believe that while our patient did not experience significant clinical improvement without PLEX, progressive organ dysfunction was likely prevented until caplacizumab was added.
In summary, our case provides additional evidence that caplacizumab with immunosuppression with rituximab, corticosteroids, and vincristine may be a feasible alternative for treatment of TTP without PLEX. This approach could be useful for treatment of patients who are unwilling or unable to accept plasma transfusion but also could also prevent the complications of PLEX including central line placement, infusion reactions, anaphylaxis, and risk for hypocalcemia. A clinical trial to evaluate use of caplacizumab and immunosuppression without plasma exchange would provide data on the safety and efficacy of this approach. In addition, our case also provides support for the use of plasma exchange with albumin in patients who cannot get plasma transfusions for emergent management, and also provides additional support for use of caplacizumab as delayed or step-up therapy.
Patient Perspective
As a patient who does not accept blood, finding out I had TTP was terrifying. When I relapsed, the reality hit that this is something I will have to deal with for the rest of my life. It's scary that my physical symptoms are minimal and only appear when the TTP has already progressed to a life-threatening point. However, having an amazing medical medical team that was knowledgeable about TTP was vital to my survival. By getting my ADAMTS13 checked, a relapse was able to be detected. Although it was caught too late, knowing that getting my ADAMTS13 levels checked on a regular basis gives me peace of mind that a relapse can be detected in the future.
Since I relapsed, finding out that there was a new medication (Cablivi®) that was a bloodless option was exciting. The Cablivi® helped my platelets increase much faster than when I had TTP the first time. The side effects were minimal and went away when I finished the medication. Although there are a lot of treatments I have to receive and have some side effects from them, I feel confident that I could successfully overcome another relapse using bloodless options that respect my religious beliefs.
References
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017; 129(21): 2836-2846.
- Amorosi E. Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine. 1966; 45: 139-159.
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991; 325(6): 393-397.
- Reverberi R, Reverberi L. Removal kinetics of therapeutic apheresis. Blood Transfus. 2007; 5(3): 164-174.
- Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016; 127(24): 3092-3094.
- Scully M, Cataland SR, Peyvandi F, Coppo P, Knöbl P, Kremer Hovinga JA, et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine. 2019; 380(4): 335-346.
- Peyvandi F, Scully M, Kremer Hovinga JA, Cataland S, Knöbl P, Wu H, et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine. 2016; 374(6): 511-522.
- Religious and Ethical Position on Medical Therapy and Related Matters. [cited 2020 April 10]; Available from: https://www.jw.org/en/medical-library/strategies-downloads/religious-and-ethical-position-medical-therapy/
- Chai W, Chaudhry A, Rabinowitz AP. Successful management of thrombotic thrombocytopenic purpura in a Jehovah's Witness without plasma exchange. J Clin Apher. 2015; 30(1): 46-49.
- Dabak V, Kuriakose P, Raman S. Successful management of a Jehovah's Witness with thrombotic thrombocytopenic purpura unwilling to be treated with therapeutic plasma exchange. J Clin Apher. 2007; 22(6): 330-332.
- Drummond MW, Green R, Callaghan T, Watson D, Simpson K. Management of a Jehovah's witness with thrombotic thrombocytopaenic purpura/haemolytic uraemic syndrome. J Clin Apher. 2000; 15(4): 266-267.
- Walia SS, Walia MS, Walia HS. Thrombotic thrombocytopenic purpura treated with vincristine in a Jehovah's witness. Asian journal of transfusion science. 2011; 5(2): 180-181.
- George JN, Sandler SA, Stankiewicz J. Management of thrombotic thrombocytopenic purpura without plasma exchange: the Jehovah's Witness experience. Blood Adv. 2017; 1(24):2161-2165.
- Kirti FIG CA. Treatment of a Jehova’s Witness with recurrent thrombotic thrombocytopenic purpura with rituximab. J Thromb Haemost. 2015; 13(Suppl 2): 473.
- Sam C, Desai P, Laber D, Patel A, Visweshwar N, Jaglal M. Pegylated bovine carboxyhaemoglobin utilisation in a thrombotic thrombocytopenic purpura patient. Transfusion medicine (Oxford, England). 2017; 27(4): 300-302.
- Baseri B, Vishwanathan S, Benasher D, Khazan M, Luhrs C, Tsai HM. Survival of a Jehovah's Witness with thrombotic thrombocytopenic purpura without using plasma: A case report and review of the literature. J Clin Apher. 2019; 34(5): 623-630.
- Chander DP, Loch MM, Cataland SR, George JN. Caplacizumab Therapy without Plasma Exchange for Acquired Thrombotic Thrombocytopenic Purpura. N Engl J Med. 2019; 381(1): 92-94.
- Lim MY, Greenberg CS. Successful Management of Thrombotic Thrombocytopenic Purpura in a Jehovah’s Witness: An Individualized Approach With Joint Decision-Making. Journal of Patient Experience.0(0):2374373519829902.
- Lim W, Vesely SK, George JN. The role of rituximab in the management of patients with acquired thrombotic thrombocytopenic purpura. Blood. 2015; 125(10): 1526-31.
- Ferrara F, Copia C, Annunziata M, Spasiano A, Di Grazia C, Palmieri S, et al. Vincristine as salvage treatment for refractory thrombotic thrombocytopenic purpura. Annals of hematology. 1999; 78(11): 521-523.
- Ziman A, Mitri M, Klapper E, Pepkowitz SH, Goldfinger D. Combination vincristine and plasma exchange as initial therapy in patients with thrombotic thrombocytopenic purpura: one institution's experience and review of the literature. Transfusion. 2005; 45(1): 41-49.
- FDA approved caplacizumab-yhdp. [cited 2019 November 10]; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approved-caplacizumab-yhdp

