Unusual course of prostate cancer in PET/MR with [68Ga] Ga-PSMA-11
Marta Maruszak*, Bartłomiej Małkowski, Paweł Waśniowski, Bogdan Małkowski
Nuclear Medicine Department, Franciszek Lukaszczyk Oncology Centre, Bydgoszcz Poland
Department of Urology, Franciszek Lukaszczyk Oncology Centre, Bydgoszcz Poland
Department of Inorganic and Analytical Chemistry, Faculty of Pharmacy, Nicolaus Copernicus University, Collegium Medicum, Bydgoszcz, Poland
Department of Diagnostic Imaging Nicolaus Copernicus University, Collegium Medicum, Bydgoszcz, Poland
Received Date: 28/04/2022; Published Date: 11/05/2022
*Corresponding author: Marta Maruszak, Nuclear Medicine Department, Franciszek Lukaszczyk Oncology Centre, dr Izabeli Romanowskiej 2 Street, 85-796 Bydgoszcz, Poland
Abstract
Effectiveness of radioguided surgery using [99mTc] Tc-PSMA in the treatment of prostate cancer metastasis in unusual location.
Keywords: Prostate cancer; Radioguided surgery; PSMA; Mesenteric adenopathy
61-year-old man after radical prostatectomy in 2009 (Gleason 3+4), radiotherapy in 2010 and three episodes of biochemical recurrence: in 2015/16- L-shaped pathological mass on S3 level- unsuccessfully treated by surgery, successfully by SRT (PSA level from 1,9 to 0,4ng/ml) in 2017/18- recurrence in sacral lymphnode (PSA 1,29 ng/ml)-unsuccessful SRT and surgery with Nanoknife, successfull RTH (PSA 0,7 ng/ml). From 2018 the patient has been under active surveillance. The follow-up procedures included PET/MR scans with [68Ga] Ga-PSMA. Scan performed in 2020 detected a focal lesion in intestinal mesentery - an unusual location for the prostate cancer metastasis [1,2,3] - originally described as possibly reactive. Along with increasing PSA levels (from 0,7ng/ml in 2019 to 7,79 ng/ml in 06.2020) the lesion uptake in subsequent studies was also significantly increasing, which suggested a focal recurrence (Figure 1). The pre-procedures for the scheduled radioguided surgery included pelvis PET/MR after intravenous 200mBq [68Ga] Ga-PSMA administration which revealed an irregular mass in the intestinal mesentery with high uptake of radiopharmaceutical, high gadolinum enhancement and restricted diffusion in MRI (Figure 2). Approximately 20 hours before the surgery patient was given intravenously 720 MBq [99mTc] Tc-PSMA. On the surgery day we performed pelvis SPECT/CT scan to validate the radiopharmaceutical uptake (Figure 3). Resection of pathological mass was supported by intraoperative radioguidance using a gamma probe. Histopathology report confirms the presence of prostate cancer cells in the resected tissue. Four weeks after the surgery, the PSA level shows full biochemical response which persists till today (decrease from 7,79ng/ml to 0,02 ng/ml), what clearly indicates the effectiveness of radioguided surgery using [99mTc] Tc-PSMA.

Figure 1: [68Ga] Ga-PSMA PET/MRI scan demonstrating focal lesion in intestinal mesentery with moderate radiopharmaceutical uptake.
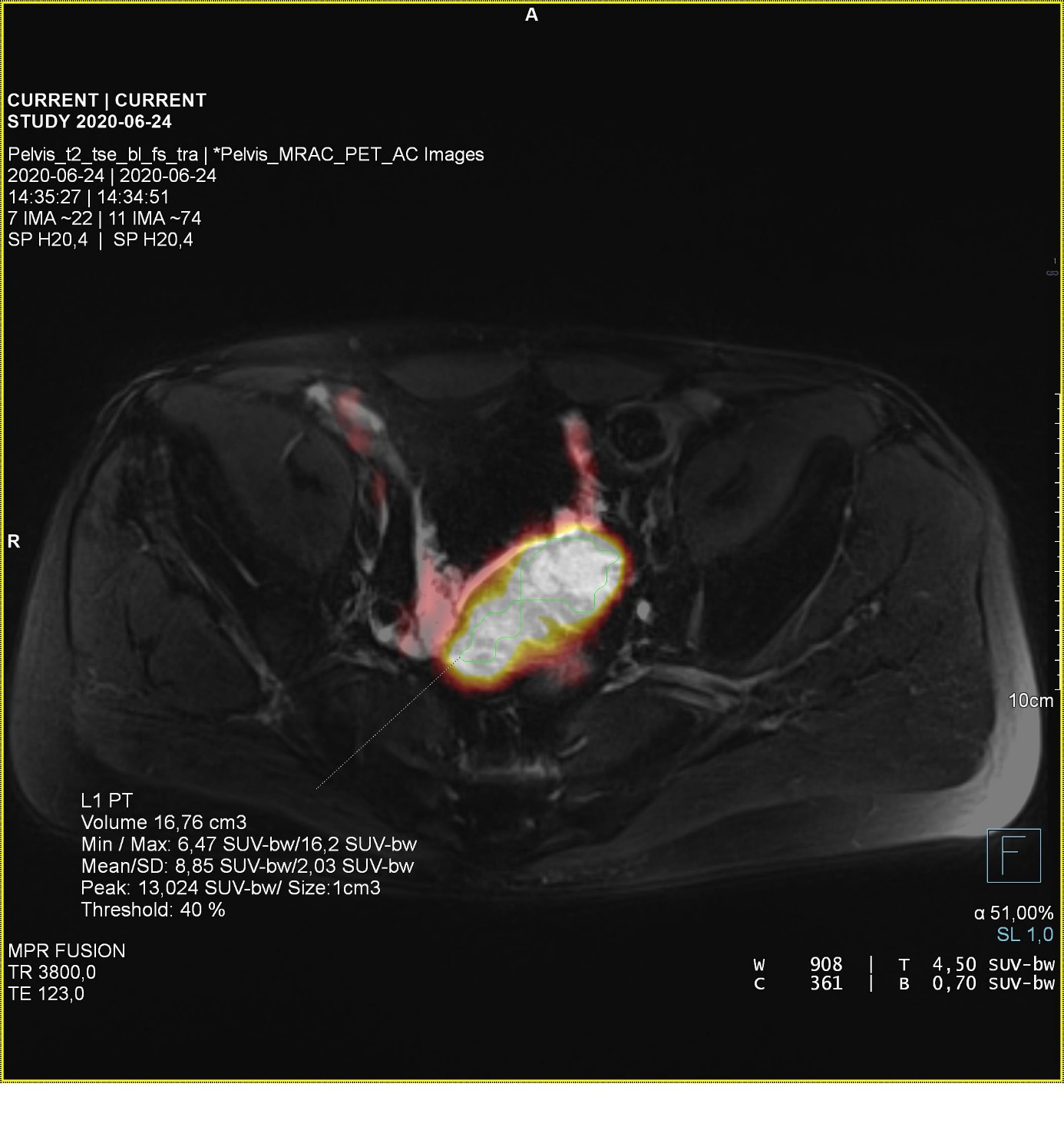
Figure 2: [68Ga] Ga-PSMA PET/MRI scan showing increased radiopharmaceutical uptake.

Figure 3: [99mTc] Tc-PSMA SPECT/CT scan revealing radiopharmaceutical uptake in lesion.
Conflicts of Interest: The authors have no conflicts of interest to declare.
Grants: The authors received no specific funding for this work.
References
- Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev, 2008; 29(4): 441-464.
- Ahmed SF, Elmantaser M. Secondary osteoporosis. Endocrine Development, 2009; 16: 170–190.
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int, 2014; 25(10): 2359-2381
- Koloszár S, Gellén J, Kovács L. The value of plasma prolactin level determination in the diagnosis of postmenopausal osteoporosis. Orv Hetil, 1997; 138(2): 71-73.
- Shibli-Rahhal A, Schlechte J. The effects of hyperprolactinemia on bone and fat. Pituitary, 2009; 12(2): 96-104.
- Di Somma C1, Colao A, Di Sarno A, Klain M, Landi ML, Facciolli G, et al. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab, 1998; 83(3): 807-813.
- Colao A, Di Somma C, Loche S, Di Sarno A, Klain M, Pivonello R, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf), 2000; 52(3): 319-327.
- Naliato EC, Farias ML, Braucks GR, Costa FS, Zylberberg D, Violante AH. Prevalence of osteopenia in men with prolactinoma. J Endocrinol Invest, 2005; 28(1): 12-17.
- Greenspan SL, Neer RM, Ridgway EC, Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med, 1986; 104(6): 777-782.
- Sartorio A, Conti A, Ambrosi B, Muratori M, Morabito F, Faglia G. Osteocalcin levels in patients with microprolactinoma before and during medical treatment. J Endocrinol Invest, 1990; 13(5): 419-422.

