Lipomatous Hypertrophy of the Interatrial Septum
Bouamoud A*, Khalloq R and Pr Asfalou I
Mohamed V Military Instruction Hospital, Rabat, Morocco
Avicenna University Hospital Rabat, Morocco
Received Date: 10/05/2025; Published Date: 17/06/2025
*Corresponding author: Bouamoud A, Mohamed V Military Instruction Hospital, Rabat ; Avicenna University Hospital Rabat, Morocco
Abstract
Primary tumors of the heart are rare, may be malignant in 25% of cases, dominated by rhabdomyosarcomas and angiosarcomas, or benign, corresponding essentially to myxomas or rhabdomyomas.
Lipomatous hypertrophy of AIS is a benign cardiac tumor, its frequency has long been poorly known since most of the available data came from autopsy series. Recent echocardiographic data have reported that lipomatous hypertrophy of the interatrial septum has become a fairly common finding, thus posing a real problem of differential diagnosis with other cardiac masses, especially malignant tumors.
We report the clinical case of an asymptomatic 66-year-old patient in whom the diagnosis of lipomatous hypertrophy of the interatrial septum was evoked in view of the typical appearance of ETT and ETO.
Through this case we will focus on the epidemiological, histological and morphological aspect of this type of mass with echocardiography, CT scan and MRI, which are sufficient to make the diagnosis of this entity without having to resort to endomyocardial biopsy.
Introduction
Primary tumors of the heart are rare, may be malignant in 25% of cases, dominated by rhabdomyosarcomas and angiosarcomas or be benign, corresponding essentially to myxomas or rhabdomyomas. Intracardiac lipomas are extremely rare benign tumors, accounting for 8.4% of primary tumors of the heart. Lipomas involving the interatrial septum are distinguished into true lipoma and lipomatous hypertrophy of the interatrial septum. These two well-defined entities are sometimes difficult to differentiate [1].
Lipomatous hypertrophy of the interatrial septum (AIS) is a rare entity, but whose anatomopathological and morphological characteristics are well known, characterized by an excessive accumulation of fat cells at the level of the septum separating the atria, achieving a non-capsulated fatty hypertrophy of the normal structures of the AIS whose thickness exceeds 20 mm. This benign tumor is very often asymptomatic and discovered by chance during echocardiography. It deserves recognition because of the differential diagnosis issues it raises [2].
Clinical Case
This is a 66-year-old patient with a cardiovascular risk factor for type 2 diabetes and a surgical history of surgery during childhood for a fracture of the right leg with osteosynthesis (non-MRI compatible equipment).
The patient suffered from varicose veins of both lower limbs that were indicated for surgery. As part of a pre-anesthesia cardiac evaluation, the patient benefited from an ETT that fortuitously objectified a mass at the level of the right atrium adjoining the interatrial septum without invasion of adjacent structures, in particular the vena cava, and without obstruction of the tricuspid flow. The ETO objectified two non-vascularized masses measuring respectively 12*20mm and 12*15 mm, sparing the oval fossa with an hourglass appearance, without invasion of the superior vena cava.
The ECG recorded a sinus rhythm at 70 beats per minute with no abnormalities, a complement by holter ECG was requested to look for arrhythmia returned normal.
Additional MRI was indicated, however the patient had compatible non-MRI equipment in his leg.
The diagnosis of lipomatous hypertrophy of the interatrial septum was retained on the typical appearance of the ET, but without confirmation on MRI. It was decided to monitor the patient with annual ETTs.
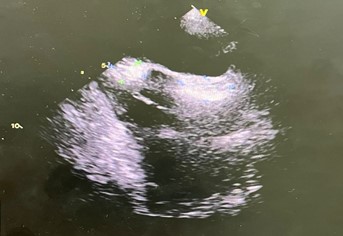
Figure 1: TEE image showing two masses attached to the interatrial septum on its right side, with a broad base of implantation, shaped like an hourglass.
Discussion
The first anatomical description of lipomatous hypertrophy of the interatrial septum (HLSI) dates back to 1964, while the first in vivo diagnosis of this tumor was made in 1982 using echocardiography imaging [2,3].
The frequency of lepromatous hypertrophy of AIS has long been poorly known since most of the available data came from autopsy series. The autopsy incidence of lipomatous hypertrophy of the AIS is 1%, whereas recent echocardiographic data have reported that lipomatous hypertrophy of the interatrial septum has become a fairly common finding with a reported incidence of 1-8% of the general population [4,6] . This difference can be explained by the technological advances in modern imaging. About 250 observations have been reported in the literature. It preferentially affects women in the elderly, obese, frequently suffering from atrial fibrillation or with a history of pulmonary disease [1,5] . According to the literature, a high incidence has been noticed in patients with metabolic disorders such as cerebrotendinous xanthomatosis or mediastino-abdominal lipomatosis and also in patients receiving long-term parenteral nutrition [3].
Histologically, the lipomatous hypertrophy of AIS is characterized by the presence of adipocytes, between which hypertrophied myocardial fibers are embedded. Occasionally, multivacuolar fat cells identical to fetal fat cells are found. The latter are considered by some authors to be the histological marker of this entity, as is the absence of a capsule distinguishing it from true cardiac lipomas [1]. Macroscopically, the tissue constituting this hypertrophy cannot be differentiated from the epicardial adipose tissue. It seems that a significant increase in epicardial fatty tissue is readily observed in these patients [2].
Anatomically, it is an accumulation of fatty tissue at the level of the interauricular septum sparing the foramen ovale responsible for a dumbbell or hourglass appearance. It is in continuity with the epicardial fat and can bulge laterally in one of the ear cavities, especially on the right, and then simulate a tumor [2].
The diagnosis of HLSI is most often fortuitous, with clinical signs absent or non-specific, as in our observation. Lipomatous hypertrophy of the AIS can, in very rare cases, become obstructive and invade the superior vena cava [2] .
Some series have reported ECG abnormalities, an alteration of the P wave is often noted, it can be negative, enlarged, hooked or biphasic. Other electrical abnormalities may be highlighted in the form of supraventricular arrhythmias (50% of cases) due to local irritation: atrial extrasystoles, paroxysmal supraventricular tachycardia, atrial tachycardia, atrial fibrillation or flutter [1]. The frequency of these arrhythmias seems to be related to the extent of hypertrophy: a septal thickness greater than 30 mm is associated with an incidence of atrial arrhythmias of more than 60% in the Shirani and Roberts series [2,8].
In echocardiography (ETT and ETO), lipomatous hypertrophy results in a thickening of the AIS respecting the oval fossa (dumbbell appearance), almost pathognomonic when the thickness exceeds 20 mm, but it can vary depending on the series from 20 mm to 62 mm, with an average of 35 mm. It constitutes an unencapsulated hyperechoic mass, very little mobile, non-circulating, with a wide implantation base, which differentiates it from the myxoma, sometimes with linear echoes. It protrudes into the right atrium, no case of protrusion in the left atrium has been reported. Echocardiography cannot, however, differentiate between fatty tissue and connective tissue [2,4].
It is therefore necessary to confirm the fatty nature of the tumor by using chest CT or cardiac MRI. The CT scan can detect the presence of fat at the level of inter-atrial thickening (density ≤ 50 HU), within a mass with clear boundaries. MRI clarifies the ultrasound data, confirms the fatty content of the tumor process and thus limits the use of invasive methods (endomyocardial biopsy). It objectifies a well-limited and non-encapsulated lesion, respecting the oval fossa and the surrounding structures, appearing as a characteristic hypersignal on the T1-weighted sequences and as a T2-weighted hyposignal, especially after fat saturation. Typically, the thickening spares the oval pit giving a classic "hourglass" or "dumbbell" appearance [5]. Gadolinium injection does not enhance the tumor [2] [1, 4] . Epicardial or mediastinal fat infiltration is often associated[5].
The Pet Scanner can objectify a hypermetabolism thus causing the "hot spot" image of the interatrial septum in HLSA and lead us to a malignant nature of the mass [6,7].
The main differential diagnosis of lipomatous hypertrophy of AIS is lipoma. This benign tumor is rarer, only 70 observations have been reported in the literature, described in young patients, sometimes in the context of tuberous sclerosis of Bourneville. It is encapsulated and can affect epicardial, endocardial and pericardial fatty tissues. Their predictive site is the left ventricle and the right atrium but can also more rarely affect the right ventricle, the left atrium and the interventricular septum. The lipoma appears on echocardiography as a hypoechoic mass when it is pericardial and hyperechoic when it affects the heart chambers [2]. Their appearance in MRI and CT is characteristic. These are homogeneous, encapsulated, fat density masses, whose high signal on the T1 and T2 sequences collapses after fat saturation. They generally do not have significant contrast intake. Septa can be visualized. The presence of tissue components within the lipoma should suggest a well-differentiated liposarcoma (extremely rare)[5] . Microscopically, these are mature fat cells without fetal cells. These lipomas are most often asymptomatic and the surgical indication is very rare, reserved for large lipomas with pericardial development [1,5].
The prognosis for lipomatous hypertrophy of the AIS is good and surgical treatment is reserved only for rare cases of invasion of the superior vena cava with hemodynamic repercussions or malignant cardiac arrhythmias, which cannot be controlled under drug treatment [1,2].
Conclusion
Lipomatous hyperplasia of AIS is a benign tumor, most often affecting overweight women over the age of 60. Its clinical manifestations are very infrequent and non-specific, its diagnosis is most often fortuitous in the presence of a homogeneous mass of DO with thickening of the AIS into ETT. In order to refine the relationships, it may be useful to carry out an ETO. This examination makes it possible to better visualize the thickening of the AIS, which respects the oval fossa, but cannot categorically affirm its lipid nature. This argument is provided by chest computed tomography and especially cardiac MRI, which make it possible to differentiate this tumor from other intracardiac masses and thus to limit the use of a more invasive procedure allowing histopathological identification [2].
References
- Ben Ghorbel I, et al. Cardiac arrhythmia revealing intracardiac lipoma. Journal of Vascular Diseases, 2004; 29(1): pp. 41-44.
- Mioulet D, et al. Lipomatous hypertrophy of the interatrial septum. 2009; 1681(2): p. Pages 61-118.
- Casado-Arroyo R, Vidal-Perez R, Maeda S. Gaining Insights Into Lipomatous Hypertrophy of the Interatrial Septum: A Step Forward∗. JACC: Case Reports, 2020; 2(14): pp. 2240-2243.
- Gopal DJ, et al. Atypical presentation of lipomatous hypertrophy of the interatrial septum: a case report: Eur Heart J Case Rep, 2019; 3(4): 1-4. doi: 10.1093/ehjcr/ytz202.
- Moskovitch G, et al. Cardiac tumors: CT and MRI aspects. Journal of Radiology, 2010; 91(9, Part 1): p. 857-877.
- Coulier B, Richelle F. Lipomatous hypertrophy of interatrial septum causing hot spot on (18)FDG PET/CT. Diagn Interv Imaging, 2019; 100(3): pp. 197-198.
- Siminiak N, et al. Changing appearance of lipomatous hypertrophy of the interatrial septum on positron emission tomography scan. Kardiol Pol, 2021; 79(9): pp. 1032-1033.

