A Successful Case of Langerhans Cell Sarcoma Treated with a Combined Chemotherapy, Surgical and Radiotherapy Approach, Followed by Autologous Stem Cell Transplantation: A Case Report and Review of the Literature
Alessia Castellino*, Roberto Soriasio, Fabrizio Giordano, Andrea Bianchi, Alessia Reali, Stefano Giaccardi, Claudia Castellino, Elisa Santambrogio1, Ambra Buschiazzo, Daniele Mattei, Nicola Mordini, Myriam Foglietta, Ivana Celeghini, Mariella Grasso, Giulio Fraternali Orcioni, Massimo Massaia, Davide Rapezzi
Division of Hematology, Santa Croce e Carle Hospital, Cuneo, Italy
Division of Pathology, Santa Croce e Carle Hospital, Cuneo, Italy
Division of Nuclear Medicine, Santa Croce e Carle Hospital, Cuneo, Italy
Division of Radiotherapy, Santa Croce e Carle Hospital, Cuneo, Italy
Division of General Surgery, Santa Croce e Carle Hospital, Cuneo, Italy
Received Date: 09/10/2021; Published Date: 04/11/2021
*Corresponding author: Alessia Castellino, MD, Division of Hematology, Santa Croce e Carle Hospital, Via Michele Coppino, 26 12100, Cuneo, CN, Italy
Abstract
Langerhans Cell Sarcoma (LCS) is a rare malignant neoplasm of the Langerhans cells, with aggressive clinical course and usually poor outcome. Since its rarity, large epidemiologic data on LCS are lacking, and most of the available knowledge derives from individual case reports. There is a lack of evidence regarding the most appropriate treatment approach: most of the treatment protocol employed remain empirical combination of surgery, radiotherapy and chemotherapy, with variable results. Here we report a case of LCS local advanced with left supraclavicular and axillar bulky mass, successfully managed with a multidisciplinary approach and a combined chemotherapy, surgery and radiotherapy treatment, followed by ASCT consolidation. Further researches will be needed to determine a systemic therapy that is effective for these patients.
Keywords: Langerhans Sarcoma; Dendritic cells neoplasm; Langerhans cell histiocytosis
Introduction
Langerhans Cell Sarcoma (LCS) is a rare malignant neoplasm of the Langerhans cells, with aggressive clinical course and usually poor outcome [1-2]. It’s characterized by rapid grow, local invasion and tendency to recur [3]. Since its rarity, large epidemiologic data on LCS are lacking, and most of the available knowledge derives from individual case reports. The most frequent sites of disease involvement described are lymph nodes (74%), skin (49%), lung (29%), spleen and bone marrow [1]. The few data available showed significantly worse prognosis in patients with advanced disease, with 5 years disease specific survival (5y-DFS) of 70% for one site, 15% for locoregional involvement, and 0% for disseminated disease, with a mean DFS of 27 months for all patients [1,3]. There is a lack of evidence regarding the most appropriate treatment approach to this complex clinical condition. Most of the treatment protocol employed remain empirical combination of surgery, radiotherapy (RT) and chemotherapy, with variable results [1,3].
Here, we reported the case of a LCS patient, successfully treated with combining a chemotherapy plus surgical plus radiotherapy approach, followed by consolidation with autologous stem cell transplantation (ASCT).
Case
M.B. was a 58 years old man, of African black descent, silent clinical history. He presented at our clinic for a left axillary swelling, grown in the last year, with persistent pain. He had no fever, night sweats, itch, but he referred a 6 kg weight loss in the previous 6 months.
His clinical examination revealed two small centimetric (cm) adenopathy supraclavicular and axillary left and a huge 10 x 8 x 15 cm left axillary, partially ulcerated mass (Figure 1a). No other enlarged lymph nodes and no hepatic or splenomegaly. The remaining objectivity was unremarkable.
Since the complete surgical exeresis of this mass was not possible, a diagnostic biopsy was performed, showing neoplastic proliferation by large and pleiomorphic tumor cells, with abundant
pale eosinophilic and xanthomata cytoplasm and bizarre, grooved nucleoli. Many of the cells exhibited multinucleation, nuclear lobulation, and high mitotic activity.
The neoplastic cells had the LC immunophenotype, and strongly expressed CLA/ CD45, CD1a and S-100. The cells were also focally positive for CD-68, CD14, Lisozime and CD163. On the other side, neoplastic cells resulted negative for CD30, ALK-1, CD-123, CD-3, CD-79a. Proliferation index Ki67 was 70-80%. BRAF mutation was negative. The biopsy specimen was diagnosed as LCS (Figure 2).
Staging showed stage II disease, with hight metabolic active multiple supraclavicular and axillary nodes and left axillary mass (SUV max 29.7) (Figure 3 a,d). Bone marrow biopsy and aspirate was performed, showing not evidence of Langerhans cell neoplasm, but, surprising, a 20-30% of infiltrate by clonal, lambda restricted, plasma cells, in a diagnosis of concomitant smouldering multiple myeloma.
Since there were no treatment criteria for the smouldering multiple myeloma, the therapeutic program was defined for the LCS only. The largest mass was not removable by surgical exeresis only and radiation treatment was not safe on an ulcerated lesion, so we decided to start with chemotherapy. One course of CHOP (cyclophosphamide, vincristine, doxorubicin and prednisone) was administered, without relevant benefits. So, we added etoposide and we continued with other 5 CHOEP (CHOP plus etoposide) courses. During the fourth chemotherapy course, CD34+ stem cell apheresis was performed.
Chemotherapy was well tolerated, without major complications. During treatment we observed a progressive reduction of the largest axillary massand a complete disappearance of the others smaller adenopathies (Figure 1b).
At the end of chemotherapy, a PET scan was performed showing a complete regression of all other adenopathies, but a persistence, even if reduced in volume, of the left axillary mass, with a Deauville Score (DS) 5 (Figure 3 b,e).
Then, the patient underwent surgical exeresis of the residual mass and a subsequent adjuvant RT. RT was given on the left axilla with 10 MV X-rays and AP-PA MLC-shaped fields of linear accelerator Varian Clinac 2100 (VARIAN) used at Radiation Oncology Department of Santa Croce Carle Hospital, Cuneo. Involved site RT was administered with a fractionated scheme of 1.8 Gy per fraction, 5 days per week, with a total dose of 30.6 Gray [4] achieving a complete remission of disease (Figure 3 c,f).
Since the patient was in good clinical condition, with a complete remission of disease, we decided to perform ASCT as consolidation treatment, conditioned with FEAM chemotherapy scheme (fotemustine, etoposide, cytarabine, melphalan).
After 9 months from ASCT, the patient is in complete remission of disease, with no sign of LCS or multiple myeloma progression.
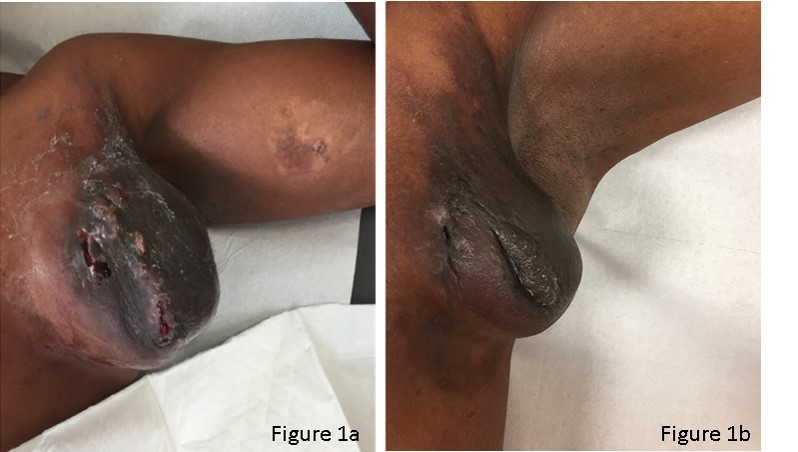
Figure 1: Macroscopic clinical presentation of patient.
Figure 1a: Clinical presentation at diagnosis.
Figure 1b: Clinical presentation at the end of chemotherapy treatment.

Figure 2: Microscopic diagnostic features.
Figure 2a and 2d: ematossiline-eosine coloured slides showing the overtly malignant cytology. The nuclear features and abundant cytoplasm point to histiocytic lesion. Nuclear pleomorphism with atypical mitosis indicated high grade disease. Some cells showed complex grooves, multinucleated cells, with crown arrangement, a key clue to the diagnosis (arrow indicated).
Figure 2b: CD1a is widely expressed on surface Langerhans cells.
Figure 2c: CD207 (Langherin) is positive.
Figure 2e: S100 widely represented, nuclear and cytoplasmatic, with variable intensity.
Figure 2f: Mib1 proliferation index is tipically high, over 70-80% in our case.

Figure 3: Positron Emission Tomography (PET) images of patients.
Figure 3a and 3d: PET images at disease staging at diagnosis.
Figure 3b and 3e: PET images at intermediate disease staging, after chemotherapy CHOEP courses, showing a complete regression of all other adenopathies, but a persistence, even if reduced in volume, of the left axillary mass, DS 5.
Figure 3c and 3f: PET at the end of treatment, before ASCT consolidation, showing complete remission of disease.
Discussion
LCS is an extremely rare disease, with generally poor prognosis. Few data are available in literature, with only two larger studies available: one systematic review [1] and a retrospective registry-based report of data from Surveillance, Epidemiology, and End Results (SEER) and National Cancer Data Base (NCDB) databases [3] in USA. There is still a lack of evidence regarding the most appropriate treatment approach for LCS. Most of the treatment protocol employed remain empirical combination of surgery, radiotherapy and chemotherapy, with variable results.
Some data suggested an advantage in radiation in the case of locoregional limited stage disease, without bone marrow or liver/splenic disease involvement [3-5]. Also, surgical resection with clear margins was associated to improved overall survival (OS) in some analysis [1], but not in others [3]. Many different systemic chemotherapy regimens have been administered in LCS patients with advanced stage disease, failing in demonstrating significant impact on OS [3, 6-8].
The case we reported showed, at diagnosis, locoregional left supraclavicular and axillar node involvement, however, the bulky ulcerated axillar mass was not surgically removable and could not receive radiation treatment upfront, since it was extensively ulcerated. For these reasons, we decided to start treatment with chemotherapy with neo-adjuvant aim. After first CHOP cycle, without any satisfactory improvement, we decided to add etoposide, basing on few data available in literature which showed good results with etoposide-based chemotherapy (such as ESHAP (etoposide plus cytarabine plus carboplatin plus methylprednisolone) [7] or EPOCH (etoposide, cyclophosphamide, vincristine, doxorubicin and prednisone) [8]). In our patients as well, we confirmed the good impact on mass clinical response to etoposide-based chemotherapy regiment, even if he did not reach a complete remission of disease with chemotherapy alone, showing the substantial chemo refractory of LCS. However, we achieved our aim to obtain a surgically removable mass.
Only few experiences in literature [10-11] reported bone marrow transplant (BMT) as consolidation treatment. However, basing on this small number of cases, BMT seemed to be one of the most reliable treatment for advanced stage LCS. Our case was only locally advanced, however, since we had stem cell storage available and our patient was in good clinical condition, we decided to perform ASCT as first remission consolidation.
To go further we can say that, in the last decade, an improving knowledge of molecular pathophysiology of LCS has opened the way for new target therapy approaches.
Genomic analysis in individual case reports described the recurrence of a missense mutation (V600E) in BRAF, cyclin-dependent kinase inhibitor-2 (CDKN2A/B) deletion, missense mutations in TP53 gene, and mutations in the NOTCH1 gene [12-14]. A report demonstrated the successful use of dabrafenib, a BRAF inhibitor, in a patient with systemic disseminated LCS (harboring BRAFV600E mutation) with progressive disease during chemotherapy [14]. This case opened the door for the promising role of target therapy in LCS as well as in other rare hematologic malignancies, uncovering future directions of research in this rare disease, in which the lack of prospective clinical trials constitutes a real unmet clinical need.
Unluckily, our patient was tested for BRAF mutation but resulted negative.
Interestingly, at the staging of disease, the bone marrow histology showed invasion by concomitant smoldering multiple myeloma. This is the only case of concomitant LCS and plasmecells neoplasm reported in literature for our knowledge. However, it is important to notice that many cases of LS have been linked to previous hematological disease. Some cases were reported arising from previous Langerhans cells histiocytosis, [1]: one case nine years after B-cell acute lymphoblastic leukemia, demonstrating an identical immunoglobulin rearrangement [10]; two further cases developed by previous hairy cell leukemias, one evolving from follicular lymphoma, with the same immunoglobulin peak, while another from chronic lymphocytic leukemia, with identical loss of the 6q23 signal on FISH [15].
In our case we do not perform clonality analysis on multiple myeloma infiltrate. However, it’s an interesting example of the hematopoietic plasticity, suggesting how much is still unknown of this rare entity.
In conclusions, we report a case of local advanced LCS with left supraclavicular and axillar bulky mass, successfully managed, thanks to an accurate pathological diagnosis, with a multidisciplinary approach with chemotherapy, surgery and radiotherapy treatment, followed by ASCT consolidation. Further researches will be needed to determine a systemic therapy that is effective for these patients, which should be based on multidisciplinary management. We think it is important to encourage physicians to publish all cases of LCS in order to improve the actual knowledge and to compile additional data that will assist in developing optimal treatments regimens for this rare and challenging disease.
Disclosures
AC, RS, FG, AB, AR, SG, CC, ES, AB, DM, NM, MF, IC, MG, GFO, MM, DR: nothing to disclose
Author Contributions
AC, RS, DR: wrote the manuscript; FG, AB: provide images; AC, RS, FG, AB, AR, SG, CC, ES, AB, DM, NM, MF, IC, MG, GFO, MM, DR: all the author reviewed and approved the manuscript.
References
- Howard JE, Dwivedi RC, Masterson L, Jani P. Langerhans cell sarcoma: a systematic review. Cancer Treat Rev 2015; 41: 320-331.
- Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Rev. 4th ed. Geneva: World Health Organization, 2017.
- Sri Harsha Tella,1 Anuhya Kommalapati,1 Karen L. Rech,2 Ronald S. Go, Gaurav Goyal. Incidence, Clinical Features, and Outcomes of Langerhans Cell Sarcoma in the United States. Clinical Lymphoma, Myeloma & Leukemia, 2019; 19(7): 441-446.
- Anna Lucas a, Eva González Barca b, Octavio Servitje c, et al. Langerhans cell sarcoma: Response to radiotherapy. Reports of Practical Oncology & Radiotherapy. 2010; 15(4): 107-109.
- Seegenschmiedt HM, Micke O, Olschewski T, et al. Radiotherapy is effective in symptomatic langerhans cell histiocytosis (LCH): long-term results of a multicenter study in 63 patients. Int J Radiat Oncol Biol Phys 2003; 57: S251.
- Uchida K, Kobayashi S, Inukai T, et al. Langerhans cell sarcoma emanating from the upper arm skin: successful treatment by MAID regimen. J Orthop Sci, 2008; 13: 89-93.
- Yoshimi A, Kumano K, Motokura T, et al. ESHAP therapy effective in a patient with Langerhans cell sarcoma. Int J Hematol 2008; 87: 532-537.
- Matsukawa T, Suto K, Miyoshi H, Oshimi K, Ohshima K, Miyagishima T. Successful treatment of an elderly Langerhans cell sarcoma patient by EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) chemotherapy. J Clin Exp Hematopathol 2018; 58: 184-187.
- Woong Do Chung, Soo Ah Im, Nak Gyun Chung, et al. Langerhans Cell Sarcoma in Two Young Children: Imaging Findings on Initial Presentation and Recurrence. Korean J Radiol. 2013; 14(3): 520-524.
- Richard Ratei, Michael Hummel, Ioannis Anagnostopoulos, et al. Common clonal origin of an acute B-lymphoblastic leukemia and a Langerhans’ cell sarcoma: evidence for hematopoietic plasticity. Heamatologica 2010; 95: 1461-1466.
- Ted Zwerdling, Eric Won, Lisa Shane, et al. Langerhans cell sarcoma: case report and review of world literature. J Pediatr Hematol Oncol. 2014; 36(6): 419-425.
- Go H, Jeon YK, Huh J, et al. Frequent detection of BRAFV600E mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014; 65: 261-272.
- Lorillon G, Mourah S, Vercellino L, et al. Sustained response to salvage therapy for dabrafenib-resistant metastatic Langerhans cell sarcoma. Ann Oncol 2016; 27: 2305-2307.
- Diamond EL, Durham BH, Ulaner GA, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature 2019; 567: 521-524.
- Chen W, Jaffe R, Zhang L, et al. Langerhans cell sarcoma arising from chronic lymphocytic lymphoma/small lymphocytic leukemia: lineage analysis and BRAF V600E mutation study. N Am J Med Sci, 2013; 5: pp. 388-391.

