ABO Blood Group System and Periodontal Disease Indices: A Cross-Sectional Study in Greek Adults
Nikolaos Andreas Chrysanthakopoulos*
Department of Pathological Anatomy, Medical School, University of Athens, Athens, Greece
Received Date: 29/06/2021; Published Date: 23/07/2021
*Corresponding author: Nikolaos Andreas Chrysanthakopoulos, Dental Surgeon (DDSc), Oncologist (MSc), Specialized in Clinical Oncology, Cytology and Histopathology, Department of Pathological Anatomy, Medical School, University of Athens, Athens, 35, Zaimi Street, PC 26 223, Patra, Greece. Tel./Fax: 0030-2610-225288. E-mail: nikolaos_c@hotmail.com, nchrysant@med.uoa.gr
-Resident in Maxillofacial and Oral Surgery, 401 General Military, Hospital of Athens, Athens, Greece
-PhD in Oncology (cand)
Abstract
Introduction: Periodontal Disease (PD) development has been associated with the presence of causative microorganisms, host immunity and risk factors, whereas the types of periodontal diseases are characterized by interactions between host and bacteria. Moreover, ABO blood groups are the most investigated erythrocyte antigen system. However, a small number of researches have been focused on the possible associations between ABO blood groups and periodontal diseases.
Methods: A cross-sectional, epidemiological study was carried out on 854 individuals, 404 males and 450 females, aged 45 to 77. The study sample was interviewed and underwent an oral and dental clinical examination. The assessment of the possible associations between several indices of PD, such as Probing Pocket Depth (PPD), Clinical Attachment Loss (CAL) and Bleeding on Probing (BOP) as dependent variables and ABO blood groups A, B, AB, and O as independent ones was carried out by using a multiple regression analysis model.
Results: Individuals with blood group A [OR= 2.94, 95% CI= 1.27-3.96] and B [OR= 2.66, 95% CI= 1.11-3.87] were significantly associated with the risk of developing deeper periodontal pockets (PPD) and worse values of attachment los (CAL) [OR= 2.42, 95% CI= 1.37-3.85] and [OR= 2.31,95% CI= 1.25-3.68], respectively. However, no significant associations were recorded between ABO blood groups and BOP [OR= 1.04, 95% CI= 0.92-1.18].
Conclusion: A significant association was revealed between A and B blood groups and deeper periodontal pockets and worse attachment loss, whereas no associations were observed between ABO blood groups and bleeding of probing.
Keywords: ABO blood group system; Periodontitis; Genetic factors; Risk factor; Adults
Introduction
Periodontal disease (PD) characterized by a multifactorial etiology and affects a large population worldwide. Dental plaque accumulation is the main etiologic factor, however genetic factors seem to play an important role in PD pathogenesis [1]. Consequently, it would be interesting to focus on the influence of genetic factors in PD patients and to investigate the possible association between those and PD. One of those factors is the ABO blood group and it would be important to investigate if the antigens of the ABO blood group have somehow increased the susceptibility to the PD. The ABO blood groups system was discovered decades ago [2] and its antigens consist biochemical indices that are expressed in several cell types including erythrocytes, gastrointestinal cells, lung epithelial cells, mucosa cells, plasma and other body fluids etc [3].
Between the presence or absence of the ABO blood group antigens and several diseases and disorders an association has been recorded, whereas those antigens also can be acted as receptors for infectious agents. Immuno-histochemical reports have demonstrated the presence of A and B antigens on spinous cells in the non-keratinized oral epithelium of blood group A and B, where basal cells express precursor structures and the more-differentiated spinous cells express the A or B antigens. Individuals with blood group O who do not have the A and B gene-coded glycosyl-transferase express a fucosylated variant (Ley) of the precursor structure [4].
An increasing number of researchers have recorded that the ABO blood group is involved in several disorders and pathological condition as has already mentioned and the possible link between ABO blood group and susceptibility to chronic disease as an example of genetic basis for family predisposition has also been investigated [5]. To be more specific an association between inherited human ABO blood group antigens with diseases such as coronary heart
disease [6], ischemic stroke [7,8] and several types of malignancies [9] including pancreatic cancer [10-12], renal cell carcinoma [13], ovarian cancer [14], colorectal cancer [15,16], gastric cancer [17,18], hematological malignancies [19] and lung cancer was recorded. However, the data on the role of ABO blood group factor in lung cancer is limited and inconsistent [2,20-23].
Despite the fact that a great number of reports have been carried out to examine the possible relationship between ABO blood group and incidence of several diseases and disorders, a small number of studies have investigated the relationship and the incidence regarding oral and dental diseases such as PD and the possible association with ABO blood group. Significant relationships have been observed between ABO blood group system and several oral diseases such as dental caries [24] salivary gland tumors [25], oral cancer [26] etc. Regarding the examined association between ABO blood group and PD, in some reports a significant association has been recorded [24,27-29,30-33]. On the contrary, in few studies no significant associations have been found [34-36]. The mentioned controversial findings could be attributed to the geographic diversity in the population groups.The purpose of the present study was to investigate the possible association of some PD indices with different ABO blood groups in a sample of Greek adults. Such an investigation may be is helpful to better understand the risk factors of PD and to predict the effective methods for its prevention and treatment.
Materials and Methods
Study design and study population sample
A cross-sectional, epidemiological study was carried out between 2019 and 2020. The study sizes were estimated considering the PD prevalence determined by Hyman et al. [37], with 90% confidence interval and relative precision 20.0%, whereas the age group was based on the World Health Organization (WHO) recommendations [38,39] for assessing disease prevalence. This procedure led to a study sample of 854 individuals [37]. The current investigation was carried out on 854 individuals,404 males and 450 females, aged 45 to 77. Participants, were out-patients of a dental and two private medical practices. The periodontal examination was carried out after the participant had been interviewed and completed a health questionnaire.
Individuals’ selection criteria
Participants should have at least 20 natural teeth excluding the 3rd molars and remaining roots in order to be included in the study [40]. The criteria of established periodontitis [41], which referred to at least 2 teeth with CAL≥ 6mm and more than one site with PPD ≥ 5mm was the main inclusion criterion for the participants. Individuals suffering from cardiovascular diseases, diabetes mellitus, acute infections, liver cirrhosis, malignancies, rheumatoid arthritis, immunosuppressed individuals because of recent transplantation or haematological malignancies and those who received medical treatment for the mentioned diseases, and general glucocorticoids were excluded from the study.
They also excluded individuals with who underwent scaling and root planing or surgical periodontal treatment within 6 months before their examination or those with prescription of anti-inflammatory or systemic antibiotics or other systemic drugs within the past 6 weeks.
Those criteria were applied in an attempt to eliminate or avoid as much possible potential confounding influences on the study indices examined. Group’s selection was based on the friendly and collegial environment in an effort to control potential confounders such as socioeconomic level, smoking, etc.
Oral and dental clinical examination
A well trained and calibrated dental surgeon carried out the dental and oral examination at the mentioned practices. The Periodontal examination consisted of probing pocket depth (PPD), Clinical Attachment Loss (CAL) and Bleeding on Probing (BOP) were measured by a William's 12 PCP probe (PCP 10-SE, Hu-Friedy Mfg.Co.Inc.,Chicago, IL, USA) at six sites (facial, lingual, disto-facial, mesio-facial, disto-lingual and mesio-lingual).
The presence of PPD was coded as [42]: -score 0: moderate periodontal pockets, 4-6.0 mm and
-score1: advanced periodontal pockets, >6.0 mm. The severity of CAL coded as [43]:
-score 0: mild,1-2.0 mm of attachment loss, and
-score 1: moderate / severe, ≥ 3.0 mm of attachment loss. PPD and CAL measurements concerned the immediate full millimeter, whereas the presence/absence of BOP was coded as:
- score 0: absence of BOP, and
-score 1: presence of BOP and deemed positive if it occurred within 15 seconds of probing.
Research Questionnaire
Individuals filled in a self-administered questionnaire that included variables such as age, gender, smoking status (active, former / no-smokers), socio-economic and educational level and data regarding their general medical history with reference to ABO blood group, medication, several chronic systemic diseases/disorders and the frequency of their dental follow- up. For establishment of the intra-examiner variance a randomly chosen sample of 171 (20%) individuals was re-examined clinically by the same dentist after 3 weeks, and no differences were recorded between the 1st and the 2nd clinical assessment (Cohen's Kappa= 0.96). During this time period no oral hygiene instructions were given to the participants.
Ethical consideration
The present cross-sectional study was not reviewed and approved by authorized committees (Ministry of Health, etc.), as in Greece only experimental studies must be approved by those Authorities. An informed consent form was obtained by the individuals who agreed to participate in the current study.
Statistical analysis
For each individual, the worst values of PPD and CAL at the six sites per tooth and the presence/absence of BOP were recorded and coded as dichotomous variables. Current and former smokers were coded as 1, individuals with a high socio-economic (income/monthly ≥ 1,000 €) and educational (graduated from University/College) level were coded as 0, males’ participants were coded as 1, and individuals that reported a regular dental follow-up were coded as 1. Age groups distribution was coded as 0, 1, 2 and 3 for ages 45-49, 50-59, 60-69 and 70+ respectively.
Univariate analysis was carried out to test the relationship between the independent variables examined and the ABO blood group, separately, by using x2 test. Multivariate regression analysis was carried out to model the associations between the dependent PD variables, and independent ones that were determined by the enter method. Adjusted Odds
Ratios (OR's) and 95% (Confidence Interval) CI were also calculated. Finally, the independent variables were included to stepwise method in order to estimate gradually the variables that showed significant associations with the dependent ones.
Statistical analysis was performed using the statistical package of SPSS ver.19.0. A p-value of less than 5% (p< 0.05) was considered significant for all statistical test conducted.
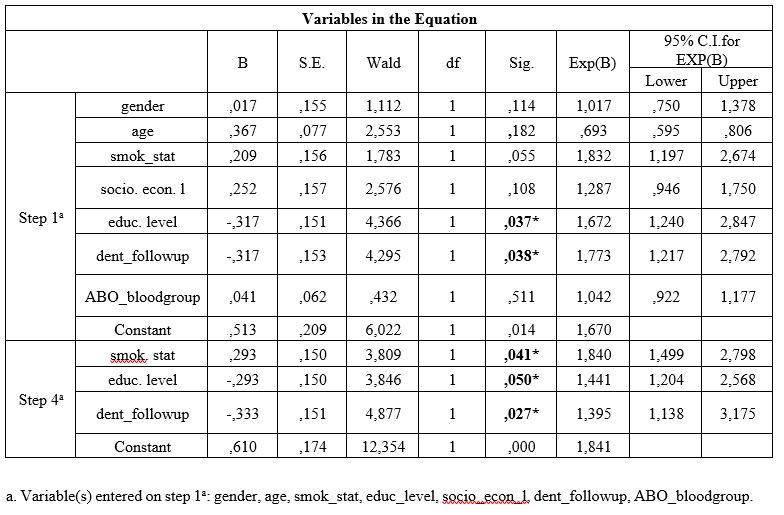
Table 1: Univariate analysis of cases and controls regarding each independent variable examined.
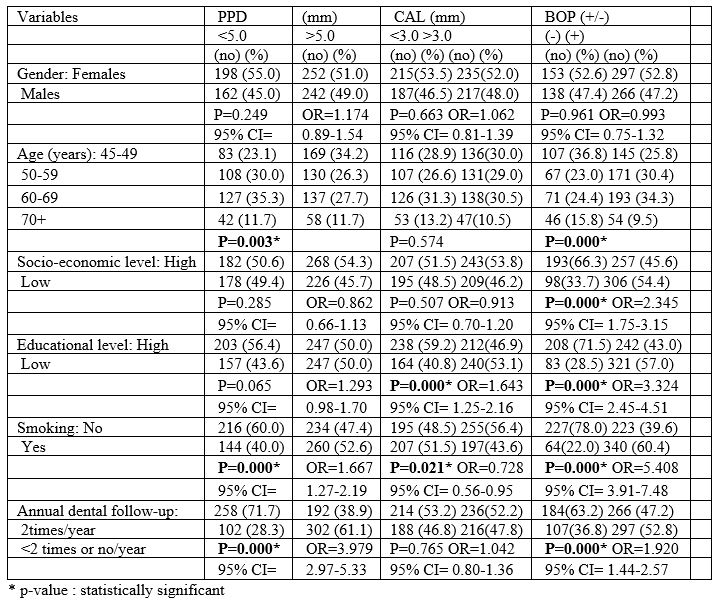
Table 2: Associations between ABO blood group and PPD.
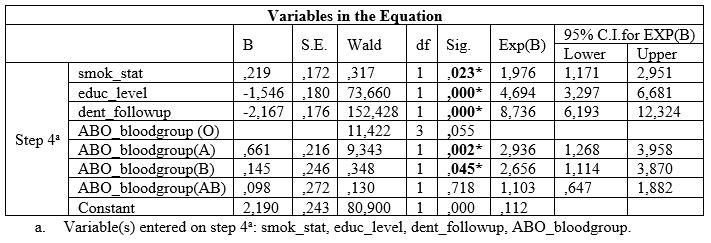
Table 3: Associations between ABO blood group and CAL.
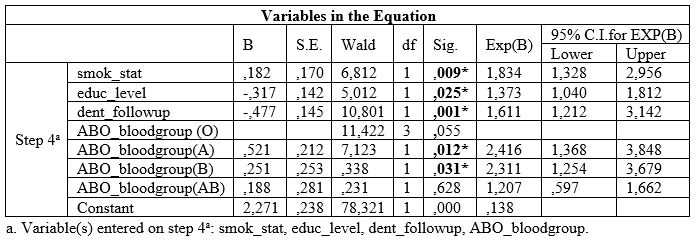
Table 4: Associations between ABO blood group and BOP.
Results
Table 1 presents univariate analysis of cases and controls regarding the examined variables. Age of the participants was statistically significantly associated with PPD and BOP, low socioeconomic level was statistically significantly associated with BOP, low educational level was statistically significantly associated with CAL and BOP, smoking was statistically significantly associated with all PD indices, irregular dental follow-up was statistically significantly associated with PPD and BOP and, ABO blood group was also statistically significantly associated with all PD indices.
Table 1 also presents unadjusted OR’s and 95% CI. After performance of the final method (step 4a) of the regression model for each PD index examined separately, it was found that low educational level, irregular dental follow-up, smoking and A and B blood groups were significantly associated with deeper periodontal pockets, and worse CAL values (Table 2 and 3). Table 2 also presents adjusted OR’s with 95% CI.
Table 4 shows that no one of the ABO blood groups were significantly associated with BOP index.
Discussion
Male gender, advanced age and low socio-economic status (SES) have been found to be significantly associated with PD, according to previous reports, and many of those have revealed more serious periodontal destruction among males compared to females [44-46]. Moreover, male gender had more severe CAL compared to females [47-50]. These differences may be explained by the facts that females usually are more aesthetically conscious, thus would be more worried about visiting the dentist whereas, males have poorer oral hygiene practices than females, access to dental care is different between both genders [51] and also could be attributed to the ignorance of oral hygiene, which is usually observed among males [52]. However, gender is a demographic variable, which may interfere with the effects of other risk factors and it must be controlled for searching the disease, as it acts as a confounder.
PD affects approximately up to half of adults over 50 years of age, one-third of adults over 30 years of age [53] and it has also been estimated that affects 30%-35% of dentate US adults [54]. Increasing age is considered as a known PD risk factor [47], and previous reports have recorded association between increasing age and CAL severity [47,50,55]. Moreover, it has been shown more severe PD based on the assessment of CAL, among older age groups compared to younger groups [56]. Aging is associated with PD, although this association could be attributed to the cumulative periodontal breakdown over time than to an age-related, intrinsic deficiency that contributes to susceptibility to PD [1].
Previous studies have recorded significant associations between socio-economic status (SES) and PD severity [48,49,57-59]. However, few studies have shown a weak association between SES and periodontitis after adjustment for oral hygiene and smoking [1,60]. Similar association has been recorded with other socio-economic parameters such as income and educational level and could be attributed to the close relation between income, educational level, and occupation [61]. Higher SES individuals wish to have better periodontal status and this is in accordance with the general belief that those individuals have healthier behaviors than individuals with lower SES [62].
Jiang et al. [63] found that risks of oral diseases increase in lower educational or academic training patients, or lack health insurance access. A higher prevalence of PD among individuals with lower educational level than among the ones with higher educational level has been observed [58, 60]. Moreover, worse PD indices in individuals lacking education, or with basic primary education has also been recorded [64]. Similar studies have revealed that low educational level was significantly associated with CAL severity [44,49,58,59], whereas individuals with lower school educational levels were 3 times more susceptible to suffer from PD that those with higher educational level [59]. Individuals who have higher educational level, and live under more favorable conditions, show better health conditions than the ones who have lower educational levels and live under less favorable conditions [65]. It is supposed that high-educated individuals take care of their own oral hygiene more than low-educated ones [66], who had difficulties with their access to social health services [64]. No significant associations were recorded among the mentioned risk factors and PD indices examined in the current research after performing of the multivariate logistic regression model, except for the educational level, as low educational level was significantly associated with the worse values of PPD, CAL and BOP.
The relationship between smoking and periodontal health has been investigated and a large amount of epidemiological, clinical and in vitro studies have provided strong evidence that smoking negatively affects periodontal health and suggest mechanisms by which this may occur [57,67,68]. Many epidemiological studies have revealed that smoking is a crucial risk factor for PD [44,48,50,55,69-72], and have shown that smoking is a significantly high-risk factor for CAL severity.
Various mechanisms have been proposed which are implicated in its pathogenesis an contributes to PD progression. This is might be related to the crucial role of smoking by affecting host immune responses [69]. However, to date it remains unclear the mechanism which explain how smoking may affect PD. It is possible that genetic susceptibility and genetic polymorphisms may explain the mentioned influence [1]. Cigarette smoking affects the inflammatory and immune responses, as well as the microvasculature [73], causes an essential destructive effect on the periodontal tissues and contributes to PD progression. It seems that cigarette smoking modifies the host’s response to the bacteria in dental plaque [74]. In the present study, a significant association between smoking and PD indices was found.
Lack of a regular dental follow-up was significantly associated with worse values of PD indices examined findings that was in agreement with the findings of a previous study [48]. Only one study recorded different findings [75]. As has already mentioned individuals with a SES are generally wish to have better periodontal health and this is in agreement with the general belief that people in higher SES have healthier oral behaviors and lifestyles than do people in lower SES [62]. Tooth brushing is essential for periodontal health maintenance as reduces accumulation of dental plaque and in turn prevent gingivitis and periodontitis [76].
The aim of the current report was to investigate the possible association between ABO blood groups and PD indices. Only few studies have investigated the relationship between ABO blood group and oral and dental diseases. Decades ago, Suk [77] suggested that ABO blood groups had an increased effect on the risk for the development of oral diseases, whereas an association between the patient’s susceptibility to dental caries and his ABO blood group was also observed [78]. In another early research [79] was found a statistical significance regarding the association between M.N. blood groups and dental caries history.
The outcomes of the current research showed that A and B blood groups were significantly associated with PD indices, such as pocket depth and attachment loss which are related to the criteria of established periodontitis. More specific, the current research showed that individuals with A and B blood groups had worse values of pocket depth (PPD) ≥ 5.00 mm and CAL ≥ 6.00mm, compared with those with O and AB blood groups, whereas no associations were observed for the other PD index examined, namely BOP. Those outcomes were in agreement with previous studies [33,80].
Vivek et al. [31] in a recent article found that the tendency for PD was least among AB blood groups individuals, whereas similar studies [29,81] recorded that blood group O may act as a predictive factor for PD development. However, the evidence of such a role requires multicenter collaborative studies that should include diverse population groups from multiple geographic regions and should explore whether there is any genetic basis for that relationship.
Similar researches showed that the mean CAL and the mean proportion of sites with CAL ≥ 3.00mm were greatest among patients of blood group B [32], and a significant association between increased incidence of Aggressive Periodontitis (AP) and blood group B, whereas in blood group O found reduced incidence of AP [28]. Moreover, some investigators demonstrated that patients with blood group B were found to be superior in the risk of developing periodontitis [33,82,83].
No significant differences between individuals with or without PD regarding ABO blood group have also been recorded [34-36,84], whereas in a study by Mortazavi et al. [85] was found that periodontitis did not show any relationship with blood groups despite the most frequent blood group had periodontitis was O.
On the contrary, Gawrzewska [86] found that individuals with blood group O had greater severity of PD, whereas individuals with blood group A had greater resistance to PD. Similarly, in a recent study was recorded a higher percentage of blood type A in patients with gingivitis and a higher percentage of blood type O in patients with periodontitis [24].
Kundu et al. [87] found that patients with Aggressive Periodontitis (AP) most frequently had group AB (60%) or group O (40%) blood type. Similar articles recorded that periodontitis was more common among patients with blood group O [27,31], and in a recent one was recorded that blood group O individuals were at a greater risk to develop Chronic Periodontitis (CP) irrespective of its severity, followed by those with blood group A, B, and AB [88]. A superior predisposition for periodontitis was also recorded in individuals with blood group O showed [89].
Nevertheless, the outcomes were controversial as were based on the type of disease and could be attributed to geographical diversity between populations. Microorganisms are involved in PD pathogenesis; however, its progression is associated with host’s risk factors, observation that shows its multifactorial nature [90]. Systemic, local, genetic and environmental factors are involved in causing PD; however, the primary factor is bacterial plaque [91].
Some individuals are at relatively high risk for developing PD as its clinical spectrum is wide. In high risk individuals, host’s factors seem to play an important role in susceptibility to periodontitis and this risk may be partly under genetic control [92]. However, in case such an association between ABO blood groups and PD can be established, it can be concluded that the presence of particular ABO blood group antigen has somehow increased the susceptibility to the disease. The association between ABO blood group and their susceptibility to chronic disease as an example of genetic basis for family predisposition has been suggested decades ago [5]. Genetic influences such as blood group antigens may act as a risk factor that affects the development and severity of the chronic periodontitis [88]. The presence or absence of certain antigens has been associated with various diseases and disorders and these antigens also act as receptors for infectious agents associated with PD. Hellstrom and Hallberg [93] found that carbohydrates act as receptors for Porphyromonas gingivalis and these carbohydrate receptors constitute the ABO antigens.
The antigens in the tissues are related to the ABO blood group, and the tissue expression is dependent on the individual’s secretor status. Secretor status is secretion of blood group antigens ABO (H), which may be influence the development of systemic oral diseases in the oral epithelium [93]. Differential secretion of blood group antigens ABO (H) in the tissues may be a factor influencing the development of systemic oral diseases [4]. Campi et al. [4] and Demir et al. [24] observed that different ABO blood groups may show significant differences in the rates of colonization of a number of periodontal pathogens that are the main etiologic factors of PD. It is very difficult to elaborate a hypothesis on why individuals with particular ABO blood group are found in increased frequency in healthy, gingivitis, and periodontitis groups, and also in various grades of periodontal involvement. However, occurrence of gingivitis and periodontitis is the result of many factors and the probable genetic influence demonstrates a small rate of multifactorial etiology of this disease. Since most of these studies were carried out on a small group of individuals, until universal epidemiological studies will be available, the decision as to whether a particular blood group has a particular immunity or susceptibility should be postponed.
Further, long-term studies with larger sample size are required to confirm this conclusion and investigate the biological plausibility to explain this association
Conclusion
A significant association was recorded between A and B blood groups and deeper periodontal pockets and worse attachment loss, whereas no associations were observed between ABO blood groups and bleeding of probing.
References
- Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996; 67(10 Suppl): 1041-1049.
- Landsteiner K. Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserumsund der Lymphe. Zentralbl Bakteriol. 1900; 27: 357-362.
- Graziano SL, Tatum AH, Gonchoroff NJ. Blood group antigen A and flow cytometric analysis in resected early-stage non-small cell lung cancer. Clin Cancer Res. 1997; 3: 87-93.
- Campi C, Escovich L, Valdés V, Garcνa Borrás S, Racca L, Racca A, et al. Secretor status and ABH antigens expression in patients with oral lesions. Med Oral Patol Oral Cir Bucal. 2007; 12: E431-434.
- Roberts JA. Blood groups and susceptibility to disease: A review. Br J Prev Soc Med. 1957; 11:107-125.
- He M, Wolpin B, Rexrode K, Manson JE, Rimm E, Hu FB, et al. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler Thromb Vasc Biol. 2012; 32: 2314-2320.
- Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. Ischemic stroke is associated with the ABO locus: The Euro CLOT study. Ann Neurol. 2013; 73: 16-31.
- Whincup PH, Cook DG, Phillips AN, Shaper AG. ABO blood group and ischemic heart disease in British men. Brit Med J. 1990; 300: 1679-1682.
- Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anticancer vaccines. Adv Exp Med Biol. 2011; 491: 369-402.
- Risch HA, Lu L, Wang J, Zhang W, Ni Q, Gao YT, et al. ABO blood group and risk of pancreatic cancer: a study in Shanghai and meta-analysis. Am J Epidemiol. 2013; 177: 1326-1337.
- Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst. 2009; 101: 424-431.
- Greer JB, Yazer MH, Raval JS, Barmada MM, Brand RE, Whitcomb DC. Significant association between abo blood group and pancreatic cancer. World J Gastroenterol. 2010; 16: 5588-5591.
Joh HK, Cho E, Choueiri TK. ABO blood group and risk of renal cell cancer. Cancer Epidemiol. 2012; 36: 528-532. - Poole EM, Gates MA, High BA, Chanock SJ, Cramer DW, Cunningham JM, et al. ABO blood group and risk of epithelial ovarian cancer within the ovarian cancer association consortium. Cancer Causes Control. 2012; 23: 1805-1810.
- Urun Y, Ozdemir NY, Utkan G, Utkan G, Akbulut H, Savas B, et al. ABO and Rh blood groups and risk of colorectal adenocarcinoma. Asian Pac J Cancer Prev. 2012;13: 6097-6100.
- Slater G, Itzkowitz S, Azar S,Aufses AH Jr. Clinicopathologic correlations of ABO and Rhesus blood type in colorectal cancer. Dis Colon Rectum. 1993; 36:5-7.
Wang Z, Liu L, Ji J, Zhang J, Yan M, Zhang J, et al. ABO blood group system and gastric cancer: a case control study and meta-analysis. Int J Mol Sci. 2012;13 :13308-13321. - Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010; 172: 1280-1285.
- Janardhana V, Propert DN, Green RE. ABO blood groups in hematological malignancies. Cancer Genet Cytogenet. 1991; 51: 113-120.
- Ulger AF, Keklik T, Kumbasar OO, Arbak P, Dermikazyk A, Gungor A, et al. Prognostic significance of blood group antigen expression of tumor tissue in lung cancer patients. Tumori 2002; 88: 395-399.
- Sanchez-Mora N, Cebollero PM, Monroy V, Herranz AM, Alvarez-Fernandez E. Expression of histo-blood group antigens in bronchial squamous metaplasia. Eur Respir J. 2007; 29: 268-272.
- Suadicani P, Hein HO, Gyntelberg F. ABO phenotypes and inflammation-related predictors of lung cancer mortality: the Copenhagen Male Study-a 16-year follow-up. Eur Respir J. 2007; 30: 13-20.
- Leon-Atance P, Moreno-Mata N, Gonzalez-Aragoneses F, Cañizares-Carreterod MA, Poblet Martíneze E, Genovés-Cresp M, et al. Prognostic influence of loss of blood group A antigen expression in pathologic stage I non-small-cell lung cancer. Arch Bronconeumol. 2012; 48: 49-54.
- Demir T, Tezel A, Orbak R, Eltas A, Kara C, Kavrut F. The Effect of ABO Blood Types on Periodontal Status. Eur J Dent. 2007; 1(3): 139-143.
- Pinkston JA, Col e P. ABO blood groups and salivary gland tumors (Alabama United States). Cancer Causes Control. 1996; 7: 572-574.
- Vijay Raghavan MR, Bailoor DN, Jhansi Rani P. Incidence of ABO blood groups in oral cancer in South Kanara District. J Ind Dent Assoc. 1986:58: 305-308.
- Koregol AC, Raghavendra M, Nainegali S, Kalburgi N, Varma S. ABO blood groups and Rhesus factor: An exploring link to periodontal diseases. Indian J Dent Res. 2018; 21: 364-368.
- Kaslick RS, Chanes AI,Tuckman MA, Kaufman B. Investigation of periodontosis with periodontitis: Literature survey and findings based on ABO blood groups. J Periodontol.1971; 42: 420-427.
- Arowojolu MO, Dosmu EB, Adingbola TS. The relationship between juvenile and non-juvenile periodontitis, ABO blood groups and haemoglobin types. Afr J Med Med Sci. 2002; 31: 249-252.
- Mansour Al-Askar. Is there an association between ABO blood grouping and periodontal disease? A literature reviews. Intervent Med Appl Sci. 2017; 9(3): 164-167.
- Vivek S, Jain J, Simon SP, Battur H, Supreetha S, Haridas R. Association of ABO Blood Group and Rh factor with Periodontal Disease in a Population of Virajpet, Karnataka: A Cross-Sectional Study. J Int Oral Health. 2013; 5(4): 30-34.
- Ali ST, Al Ghamdi. Association between ABO Blood Groups and Severity of Chronic Periodontitis. JKAU: Med Sci. 2009; 16(3): 31-41.
- Pai GP, Dayakar MM, Shaila M, Dayakar A. Correlation between “ABO” blood group phenotypes and periodontal disease: Prevalence in South Kanara district, Karnataka state, India. J Indian Soc Periodontol. 2012; 16: 519-523.
- Barros L, Witkop CJ. Oral and genetic study of Chileans 1960 III. Periodontal disease and nutritional factors. Arch Oral Biol. 1963; 8: 195.
- Frias MT, Lopez NJ. No association between secretor status of ABO blood group antigens and juvenile periodontitis. Acta Odontol Latinoam. 1994; 8(2): 9-15.
- Pradhan AC, Chawla TN, Samuel KC, Pradhan S. The relationship between periodontal disease and blood groups and secretor status. J Periodontal Res. 1971; 6: 294-300.
- Hyman JJ, Reid BC. Epidemiologic risk factors for periodontal attachment loss among adults in the United States. J Clin Periodontol. 2003; 30: 230-237.
- World Health Organization: Oral health surveys: basic methods. (4th Edn). Geneva: WHO; 1997: 47p.
- Lwanga SK, Lemeshow S. Sample size determination in health studies. A practical manual. Geneva: WHO; 1991.
- Machuca G, Segura-Egea JJ, Jimenez-Beato G, Lacalle JR, Bullón P. Clinical indicators of periodontal disease in patients with coronary heart disease: A 10 years longitudinal study. Med Oral Patol Oral Cir Bucal. 2012; 17: e569-574.
- Machtei EE, Christersson LA, Grossi SG, et al: Clinical criteria for the definition of ‘‘established periodontitis.’’ J Periodontol. 1992; 63: 206-214.
- Knowles J, Burgett F, Morrison E, Nissle R, Ramfjord S. Comparison of results following three modalities of periodontal therapy related to tooth type and initial pocket depth. J Clin Periodontol. 1980; 7: 32-47.
- Wiebe CB, Putnins EE: The periodontal disease classification system of the American Academy of Periodontology-an update. J Can Dent Assoc. 2000; 66: 594-597.
- Almerich-SillaJM, Almiñana-Pastor PJ, Boronat-Catalá M, BellotArcís C, Montiel Company JM. Socioeconomic factors and severity of periodontal disease in adults (35-44 years). A cross sectional study. J Clin Exp Dent. 2017; 9: e988-994.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause.2008; 15: 270-275.
- Mundt T, Schwahn C, Mack F, Polzer I, Samietz S, Kocher T, et al. Risk indicators for missing teeth in working-age Pomeranians-an evaluation of high-risk populations. J Publ Health Dent. 2007; 67: 243-249.
- Bouchard P, Boutouyrie P, Mattout C, Bourgeois D. Risk assessment for severe clinical attachment loss in an adult population. J Periodontol. 2006; 77: 479-489.
- Susin C, Dalla Vecchia CF, Oppermann RV, Haugejorden O, Albandar JM. Periodontal attachment loss in an urban population of Brazilian adults: effect of demographic, behavioral, and environmental risk indicators. J Periodontol. 2004; 75: 1033-1041.
- Chrysanthakopoulos NA. Epidemiological risk factors for periodontal pockets and clinical attachment loss among Greek adults. Dent Craniofac Res. 2018; 1(3): 114.
- Amran AG, Alhajj MN, Amran AN. Prevalence and Risk Factors for Clinical Attachment Loss in Adult Yemenis: A Community-Based Study in the City of Dhamar. Am J Health Res. 2016; 4(3): 56-61.
- Roberts-Thomson KF, Stewart JF. Access to dental care by young South Australian adults. Aust Dent J. 2003; 48: 169-174.
- Christensen LB, Petersen PE, Krustrup U, Kjøller M. Self-reported oral hygiene practices among adults in Denmark. Com Dent Health. 2003; 20: 229-235.
- Albandar JM. Periodontal diseases in North America.Periodontol. 2002; 29: 31-69.
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States,1988-1994. J Periodontol. 1999; 70: 13-29.
- Rao SR, Thanikachalam S, Sathiyasekaran BWCS, Vamsi L, Balaji TM, Jagannathan R. Prevalence and Risk Indicators for Attachment Loss in an Urban Population of South India. Oral Health Dent Manag. 2014;13: 60-64.
- Tanner AC, Kent R Jr, Van Dyke T, Sonis ST, Murray LA. Clinical and other risk indicators for early periodontitis in adults. J Periodontol. 2005; 76: 573-581.
- Holtfreter B, Schwahn C, Biffar R, Kocher T. Epidemiology of periodontal diseases in the Study of Health in Pomerania. J Clin Periodontol. 2009; 36: 114-123.
- Torrungruang K, TamsailomS, Rojanasomsith K, Sutdhibhisal S, Nisapakultorn K, Vanichjakvong O, et al. Risk indicators of periodontal disease in older Thai adults. J Periodontol. 2005; 76: 558-565.
- Borrell LN, Burt BA, Warren RC, Neighbors HW. The role of individual and neighborhood social factors on periodontitis: the third National Health and Nutrition Examination Survey. J Periodontol. 2006; 77: 444-453.
- Teng HC, Lee CH, Hung HC, Tsai CC, Chang YY, Connie Yang YH, et al. Life style and psychosocial factors associated with chronic periodontitis in Taiwanese adults. J Periodontol. 2003; 74: 1169-1175.
- Ismail AL, Eklund AS, Burt BA, Calderone JJ. Prevalence of deep periodontal pockets in New New Mexico adults aged 27 to 74 years. J Public Health Dent. 1986; 46: 199.
- Stephen LI, Steven AS. Class- The Ignored Determinant of the Nation’s Health. N Engl J Med. 2004; 351: 1137-1142.
- Jiang Y, Okoro CA, Oh J, Fuller DL. Sociodemographic and health related risk factors associated with tooth loss among adults in Rhode Island. Prev Chronic Dis. 2013; 10: E45.
- RamírezMaya JC, LoperaNS,López AP, Agudelo-Suárez AA, Botero JE. Periodontal disease and its relationship with clinical and sociodemographic variables in adult patients treated in a service/ teaching institution. Rev Odontol Mexicana. 2017; 21: e160-e167.
- Academy of Periodontology (AAP): Epidemiology of periodontal diseases. J Periodontol. 2005; 67:1406-1419.
- Astrøm AN, Rise J. Socio-economic differences in patterns of health and oral health behaviour in 25-yearold Norwegians. Clin Oral Invest. 2001; 5: 122-128.
- Kubota M, Tanno-Nakanishi M, Yamada S, Okuda K, Ishihara K. Effect of smoking on subgingival microflora of patients with periodontitis in Japan. BMC Oral Health. 2011; 11: 1.
- Borrell LN, Papapanou PN. Analytical epidemiology of periodontitis. J Clin Periodontol. 2005; 32: 132-158.
- Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors-tobacco smoking. J Clin Periodontol. 2005; 32: 180-195.
- Vouros ID, Kalpidis CDR, Chadjipantelis T, Konstantinidis AB. Cigarette smoking associated with advanced periodontal l destruction in a Greek sample population of patients with periodontal disease. Intern Acad Periodontol. 2009; 11: 250-257.
- Bergstrom J. Periodontitis and smoking: an evidence-based appraisal. J Evid Based Dent Pract. 2006; 6: 33-41.
- Paquette DW, Brodala N, Nichols TC. Cardiovascular disease, inflammation, and periodontal infection. Periodontol 2000. 2007; 44: 113-126.
- Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004; 75: 16-22.
- Ozcaka O, Bicakci N, Pussinen P, Sorsa T, Kose T, Buduneli N.Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis.2011; 17: 68-76.
- Ogawa H, Yoshihara A, Hirotomi T, Ando Y, Miyazaki H. Risk factors for periodontal disease progression among elderly people. J Clin Periodontol. 2002; 29: 592-597.
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States; findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000; 71: 743-75l.
- Suk V. Über die beziehung zwischen gesunden Zähnen und den Zerfall und die Pflege der Zähne bei den weissen. pisy lék Fak Masaryk Univ. 1930: 125.
- Aitchison J, Carmichael AF. The relationship between the ABO blood mutations and dental caries. Dent Pract. 1962; 13: 93-95.
- O’Rark WL, Leyschon C. Dental caries prevalence as related to blood groups. J Dent Res. 1963; 42: 1530.
- Kaslick RS, West TL, Chasens AI. Association between ABO blood groups, HL-A antigens and periodontal diseases in young adults: a follow-up study. J Periodontol. 1980; 51(6): 339-342.
- Offenbacher S: Periodontal diseases: Pathogenesis. Ann Periodontol. 1996;1: 821-878.
- Serio FG, Duncan TB. The Pathogenesis and Treatment of Periodontal Disease. Academy of Dental Therapeutics and Stomatology. PennWell Public. 2009: 1-12.
- Singh MP, Mehta A, Bhatia A, Aditya. ABO blood groups and periodontal status: is there any link? A clinical study. J Indon Dent Assoc. 2011; 5(11): 1128-1131.
- Mansour Al-Askar, Hani S AI Moharib, Razan Alqeely, Arwa A Talakey, Hamad Alzoman, Abdulmonem Alshihri. The relationship between Periodontal Disease and ABO blood groups: A Cross-Sectional Study. Oral Health Prev Dent. 2021;19: 295-300.
- Mortazavi H, Lotfi G, Fadavi E, Hajian S, Baharvand M, Sabour S. Is ABO blood group a possible risk factor for periodontal disease? Dent Hypotheses. 2015; 6: 14-18.
- Gawrzewska B. ABO, Rh and MN blood groups systems and ABH group factors in saliva as related to parodontal diseases. Czas Stomatol. 1975; 28(10): 1007-1014.
- Kundu D, Bandyopadhyay P, Nair V, Chowdhury M, Mukherjee S, Nayek M. Aggressive periodontitis: A clinico-hematological appraisal. J Indian Soc Periodontol. 2014; 18: 166-171.
- Diana Mostafa, Essam I Elkhatat, Pradeep Koppolu, Muna Mahgoub, Esam Dhaifullah, Ahmed Hussein Hassan. Correlation of ABO Blood Groups and Rh Factor with The Severity of Generalized Chronic Periodontitis: Across Sectional Study in Riyadh, Saudi Arabia. Maced J Med Sci. 2019; 7(4): 617-622.
- Anup P, Siddhartha V, Girish S, Keshava A, Sameer Z, Vishwajeet K. ABO and Rh blood group system and periodontal disease-A Prevalence Study. Brit J Med Med Res. 2016; 16(5): 1-6.
- Kinane DF, Bartold PM. Clinical relevance of host responses of periodontitis. J Periodontol. 2000; 43: 278‑293.
- Novak MJ, Novak KF. Chronic Periodontitis. In: Michael G. Newmann, Henry H. Takei, Perry R. Klokkevold, Fermin A. 2007. Carranza, editors. Carranza’s Clinical Periodontology. 10th ed. St. Louis Missouri: W.B. Saunders Company, p.494-499.
- Hart TC, Kornman KS. Genetic factors in the pathogenesis of periodontitis. Periodontol 2000. 1997; 14: 202-215.
- Hellström U, Hallberg EC, Sandros J, Rydberg L, Bäcker AE. Carbohydrates act as receptors for the periodontitis associated bacterium Porphyromonas gingivalis: a study of bacterial binding to glycolipids. Glycobiology. 2004; 14: 511-519.

