Pericardial-Esophageal Fistula: A Severe Complication After Radiofrequency-Balloon Ablation of Atrial Fibrillation
Federica Torri1, Andrea Agbariah1,2,*, Matthias Unterhuber1, Massimiliano Manfrin1, Maria Angela Deserio1, Piercarlo Farris3 and Livio Bertagnolli1
1Department of Cardiology, San Maurizio Regional Hospital of Bolzano, Bolzano, Italy
2Department of Cardiology, Azienda Ospedaliera Universitaria di Verona, Verona, Italy
3Department of Gastroenterology, San Maurizio Regional Hospital of Bolzano, Bolzano, Italy
Received Date: 29/03/2025; Published Date: 13/05/2025
*Corresponding author: Andrea Agbariah, Department of Cardiology, San Maurizio Regional Hospital of Bolzano, Bolzano, Italy; Department of Cardiology, Azienda Ospedaliera Universitaria di Verona, Verona, Italy
Abstract
We report the first case to our knowledge of a pericardial-esophageal fistula (PEF) following radiofrequency (RF) balloon ablation with the Helio-Star catheter for persistent atrial fibrillation, despite normal esophageal temperature monitoring (maximum 37.6°C). A 72-year-old man developed PEF 19 days post-procedure, presenting with chest pain, pericardial effusion, and pneumopericardium. Urgent endoscopic stenting and pericardiocentesis were performed, resulting in recovery. This case highlights that even with Helio-Star RF ablation and esophageal temperature monitoring, PEF can occur, emphasizing the importance of early recognition and prompt management of this rare but serious complication.
Keywords: Atrial fibrillation ablation; Esophageal injury; Pericardial-esophageal fistula; Radiofrequency ablation; Esophageal temperature monitoring
Introduction
Atrial Fibrillation (AF) ablation is a generally well-tolerated procedure with a low risk of complications for most patients. One of the most feared complications is esophageal injury, the incidence of which varies between 0.04 and 0.2% [1-3]. Nonspecific symptoms may cause a late diagnosis, resulting in a poor prognosis.
To limit the risk, an esophageal temperature probe is used to monitor any thermal rise in the esophagus and discontinue the delivery of radiofrequency [4,5]. We reported the first case to our knowledge of Pericardial-Esophageal Fistula (PEF) after Radiofrequency (RF)-balloon ablation for AF without a rise in esophageal temperature over 39° C in a patient, who survived after prompt diagnosis and endoscopic management through esophageal stenting.
Case Report
A 72-year-old male patient was admitted to our hospital to undergo ablation of persistent AF with RF balloon technology (Helio-Star). The patient had a history of highly symptomatic persistent AF unresponsive to antiarrhythmic therapy and arrhythmia-induced cardiomyopathy with left ventricular dysfunction. The procedure was performed in deep sedation with Midazolam and Pethidine. A quadripolar catheter (EasyFinderTM, MicroPort®, Shanghai, China) was placed in the coronary sinus. After the transseptal puncture, we selectively mapped the pulmonary veins with the 20 mm Lassostar spiral catheter, and by using the 28 mm RF Helios-Star catheter (CARTO®; Johnson & Johnson, New Brunswick, NJ, USA) we performed antral pulmonary vein isolation (Figure 1). A temperature probe (Esotherm Multi 5, Fiab, FI) within the esophagus was used to visualize its course and to monitor luminal esophageal temperature. Ablation parameters aligned with the company's specifications (15 Watt and 55°C for 60 seconds in the anterior Wall and 20 seconds in the posterior Wall for every delivery or less if the intra-esophageal temperature increased to >38°C). During ablation, the maximum intra-esophageal temperature reached 37.6°C.
After two days the patient complained of atypical pericarditis-like chest pain, so we started ibuprofen and titrated the proton pump inhibitor (pantoprazole 40 mg) from once (our standard protocol 4-6 weeks after the procedure) to twice daily. The echocardiogram showed no pericardial effusion. Nineteen days after ablation, the patient presented to the emergency department for sudden onset of acute retrosternal pain. After a pathological chest X-ray, the patient underwent a chest CT with evidence of pericardial effusion and pneumopericardium (Figure 2).
The urgent endoscopy revealed the presence of a PEF. The patient underwent an emergency pericardiocentesis with gastric material aspirated from the pericardium, pericardial cavity lavages with the physiological solution in the following days, and a long esophageal stent placement under endoscopy (Figure 3).
He was then left fasting for 10 days and subsequently resumed slowly feeding. During the hospital stay, antibiotics (amoxicillin/clavulanic acid and vancomycin) and fluconazole were administered, subsequently, the patient’s conditions improved, and signs of infection declined. After 28 days, the esophageal stent was removed, and complete healing of the previous perforation could be documented. The antibiotics were stopped, and the patient was discharged. The patient is still in good condition two months after the acute event.
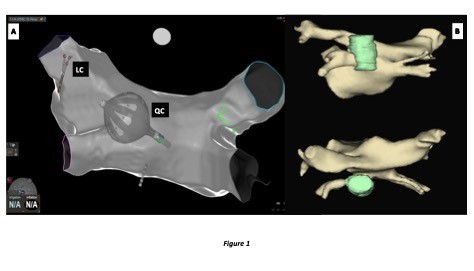
Figure 1:
A. Cranial postero-anterior projection of an anatomical map of the left atrium with the Lassostar Catheter (LC) in the left superior pulmonary vein and the Quadripolar Catheter (QC) in the coronary sinus. The projection acquired by the CARTO3 Navigation System shows that the ablation was performed in the posterior wall of the left atrium adjacent to the esophagus
B. CT 3D reconstruction of the relationship of the left atrium (yellow) and esophagus (green) in a postero-anterior view (above) and cranial view (below)
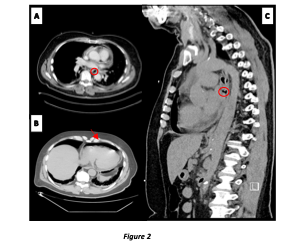
Figure 2: Imaging of PEF. Chest CT findings.
A. Axial view. Esophageal leak in the pleural space (circle)
B. Axial view. Pneumopericardium (arrow)
C. Sagittal view. Esophageal leak in the pleural space (circle)
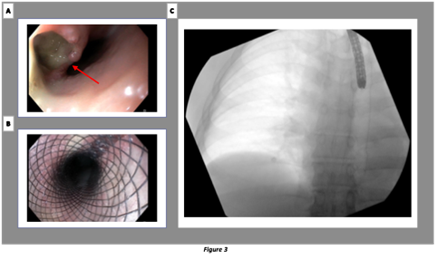
Figure 3: Pericardial-esophageal fistula treatment. Endoscopic repair has shown to be more effective for PEF than for atrial-esophageal fistula (AEF) where the outcome is poor due to the persistent communication of the open atrial end and continuation of embolization and sepsis even after stent positioning [14,15]
A. Evidence of esophageal fistula (arrow)
B. Esophageal stent placed
C. Chest X-Ray. Esophageal stent placed
Discussion
One of the most feared complications of atrial fibrillation ablation is esophageal fistula, the pathogenesis of which is still not completely clear. Certainly, the anatomical proximity between the posterior wall of the atrium and the esophagus plays a decisive role. Several theories have been proposed for its formation, including direct thermal insult leading to an inflammatory cascade resulting in esophageal erosion until the formation of the fistula [6]. This pathogenesis also explains how the onset of the lesion is later than ablation.
Symptoms are often characterized by chest pain, dysphagia, and fever and appear within a time window from ablation between 7–35 days [7,8].
Several methods have been proposed to limit the risk of this complication, including reducing the energy in the posterior wall [9], reducing the delivery time, and controlling the temperature rise in the esophagus using an esophageal tube during RF delivery or cryoablation [5]. However, in our case, temperature control was insufficient to prevent the lesion.
The pathogenesis of the complication in our patient appears unclear to date. We hypothesize that the patient's age and fragility favored esophageal thermal damage. Ischemic esophageal ulcerations could have developed in the days following the ablation with a microscopic process related to the reflux of gastric acid and potentially facilitated by damage to peri-esophageal nerves and esophageal motility, use of ibuprofen and delay of the healing process (secondary to transmural damage of the vasculature and also to decreased pain sensation and inflammation) [10,11].
According to Kapur et al., the esophageal temperature delta during the procedure plays a role in the genesis of esophageal lesions: a 1°C delta in endoluminal temperature increases the odds by 1.4 [11].
Esophageal temperature monitoring (ETM) does not reduce the incidence of endoscopically detected esophageal lesions during RF AF ablation. The higher energy delivered to the posterior wall is likely due to a false sense of safety, which may explain the lack of benefit of ETM [12].
In a study by Barbhaiya, esophageal monitoring was implemented in 90% of cases; however, no specific monitoring technique was associated with improved survival [13].
Conclusion
Pericardial-esophageal fistula is a severe complication that should never be underestimated, even when the esophageal temperature remains below 39°C during ablation. If not promptly recognized and treated, it can be life-threatening and represent a highly traumatic event for the patient. As Dr. Schoene et al. demonstrated, the increased esophageal temperature does not correlate with the risk of esophageal fistula and is therefore an unreliable parameter for its prevention [9].
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Cappato R, Calkins H, Chen S-A, et al. Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circulation, 2005; 111: 1100–1105.
- Cappato R, Calkins H, Chen S-A, et al. Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circ: Arrhythmia and Electrophysiology, 2010; 3: 32–38.
- Dagres N, Hindricks G, Kottkamp H, et al. Complications of Atrial Fibrillation Ablation in a High‐Volume Center in 1,000 Procedures: Still Cause for Concern? Cardiovasc electrophysiol, 2009; 20: 1014–1019.
- Singh SM, d’Avila A, Doshi SK, et al. Esophageal Injury and Temperature Monitoring During Atrial Fibrillation Ablation. Circ: Arrhythmia and Electrophysiology, 2008; 1: 162–168.
- Liu E, Shehata M, Liu T, et al. Prevention of esophageal thermal injury during radiofrequency ablation for atrial fibrillation. J Interv Card Electrophysiol, 2012; 35: 35–44.
- Grubina R, Cha Y, Bell MR, et al. Pneumopericardium Following Radiofrequency Ablation for Atrial Fibrillation: Insights into the Natural History of Atrial Esophageal Fistula Formation. Cardiovasc electrophysiol, 2010; 21: 1046–1049.
- Han H-C, Ha FJ, Sanders P, et al. Atrioesophageal Fistula: Clinical Presentation, Procedural Characteristics, Diagnostic Investigations, and Treatment Outcomes. Circ: Arrhythmia and Electrophysiology, 2017; 10: e005579.
- Nair KKM, Danon A, Valaparambil A, et al. Atrioesophageal Fistula: A Review. J Atr Fibrillation, 2015; 8: 1331.
- Schoene K, Arya A, Grashoff F, et al. Oesophageal Probe Evaluation in Radiofrequency Ablation of Atrial Fibrillation (OPERA): results from a prospective randomized trial. EP Europace, 2020; 22: 1487–1494.
- Deneke T, Sonne K, Ene E, et al. Esophagopericardial Fistula. JACC: Case Reports, 2021; 3: 1136–1138.
- Kapur S, Barbhaiya C, Deneke T, et al. Esophageal Injury and Atrioesophageal Fistula Caused by Ablation for Atrial Fibrillation. Circulation, 2017; 136: 1247–1255.
- Salihu A, Lu H, Maurizi N, et al. Prevention of esophageal lesions during atrial fibrillation catheter ablation using esophageal temperature monitoring: A systematic review and meta‐analysis. Pacing Clinical Electrophis, 2024; 47: 614–625.
- Barbhaiya CR, Kumar S, Guo Y, et al. Global Survey of Esophageal Injury in Atrial Fibrillation Ablation. JACC: Clinical Electrophysiology, 2016; 2: 143–150.
- Leung LWM, Akhtar Z, Sheppard MN, et al. Preventing esophageal complications from atrial fibrillation ablation: A review. Heart Rhythm, 2021; 2: 651–664.
- Shalaby A, Refaat M, Sebastien G, et al. Conservative management of pericardial–esophageal fistula complicating robotic atrial fibrillation ablation. Heart Rhythm, 2011; 8: 905–908.

