One or Two Categories for Stage 1 Testicular Germ Cell Tumors
Finn Edler von Eyben*
Center of Tobacco Control Research, Denmark
Received Date: 20/03/2025; Published Date: 06/05/2025
*Corresponding author: Finn Edler von Eyben, Center of Tobacco Control Research, Birkevej 17, 5230 Odense M, Denmark
We have two main treatment options for clinical stage 1 testicular germ cell tumors (CS1 TGCT): non-risk-adapted surveillance or risk adapted treatment. Low-risk patients are treated with surveillance whereas high-risk patients are treated with cisplatin-based chemotherapy, in recent years with one or two courses of bleomycin, etoposide, and cisplatin (BEP). Correspondingly it seems to be sufficient with one or two categories for CS1 TGCT. For patients with CS1 seminoma, Denmark defined high-risk by large tumor volume of the primary seminoma. High risk patients were patients with high-volume CS1 seminoma and were and were given adjuvant external beam, radiation therapy (EBRT) for retroperitoneal lymph nodes and ipsilateral lymph nodes. Generally, patients with CS1 TGCT had excellent 5-year overall survival irrespective of the treatment option used for the patients, provided the patients followed the scheme for postoperative follow-up examinations and were treated when the follow-up showed a relapse.
For most centers, patients with CS1 non-seminomatous germ cell tumors, a few pathological features have been used to define high-risk: presence of embryonal carcinoma and lymphovascular invasion. A large study of 453 Danish patients with CS1 NSGCT followed with non-risk-adapted surveillance for a median follow-up period of 6.4 years [1] added two features, hilar invasion and tumor volume. The combinations of risk factors separated 8 groups where the cumulative rate of relapse varied from 5% for patients with four negative risk factors to 85% for patients with four positive risk factors. Table 1 shows how three factors pointed out low and high risk.
Another Danish study [2] with non-risk-adapted surveillance evaluated risk factors for relapse for 924 patients with CS1 seminoma followed for median 6.1 years. 148 (16%) patients relapsed. In multivariate analysis, testicular hilum, lymphovascular invasion, and elevated levels of human gonadotropin and lactate dehydrogenase were significant risk factors. The estimated 5-year rate of relapse varied from 6% for patients with four negative risk factors to 62% for patients with four positive risk factors.
Combined CS1 seminoma and CS1 non-seminomatous germ cell tumors share two risk factors, and per definition TGCT patients with combination of seminoma and embryonal carcinoma elements are classified as nonseminomatous germ cell tumors. Further only a small group of patients with CS1 seminoma had a risk of relapse >50%. Serum tumor markers are typically associated with nonseminomatous germ cell tumors: serum alfa fetoprotein is typically raised in NSGCT patients with yolk sac tumor elements and human chorionic gonadotropin is typically raised in NSGCT patients with choriocarcinoma elements. Nevertheless, pretreatment serum tumor markers were significant risk factors for CS1 seminoma but not for CS1 NSGCT.
Typically, high-risk patients with CS1 NSGCT are given adjuvant chemotherapy with one or two courses of BEP. Adjuvant treatment reduces the risk from 50% to less than 3%. However, the adjuvant treatment is also overtreatment for up to half of the high-risk patients.
In addition, two major staging systems, American Joint Committee on Cancer (AJCC) and Union on International Cancer Control (UICC) Tumor, Node, Metastases (TNM), stratifies CS1 TGCT in four pathological groups where pT1 is tumors limited to the testis, pT2 tumors with lymphovascular invasion, pT3 tumors with spermatic cord invasion, and pT4 tumors with scrotal invasion [3,4]. The Danish studies agree on the prognostic role of pT1 and pT2 but do not support the prognostic role of pT3 and pT4. It seems possible that the pT3 and pT4 categories reflect anatomic extent more than prognostic significance. The two classifications suggest that the anatomic spread of the CS1 NSGCT is more prognostic significant than well-established risk factors like present embryonal carcinoma.
Most patients with TGCT have clinical stage 1. For metastatic TGCT, the TNM classification is based on an analysis of an Internation Germ Cell Collaborative Group (IGCCCG) study of 5000+ patients [5]. An update analysis by the IGCCCG update study group confirmed the prognostic analysis from 1997 [6-8]. So combined, the TNM classification of metastatic TGCT is based on more than 10 000 patients from two periods of combination cisplatin-based chemotherapy. A similar collaboration effort seems to be warranted for patients with CS1 TGCT. So far, I have not found a study of risk-adapted treatment for CS1 TGCT that based risk on pT3 and pT4. A new collaborative effort may elucidate whether the new Danish analyses can be reproduced in a large-scale collaboration and whether the two categories of advanced local spread in the AJCC and TNM classifications may be important for the prognosis of CS1 TGCT.
Table 1: Risk factors for patients with NS1 NSGCT in the Wagner study.
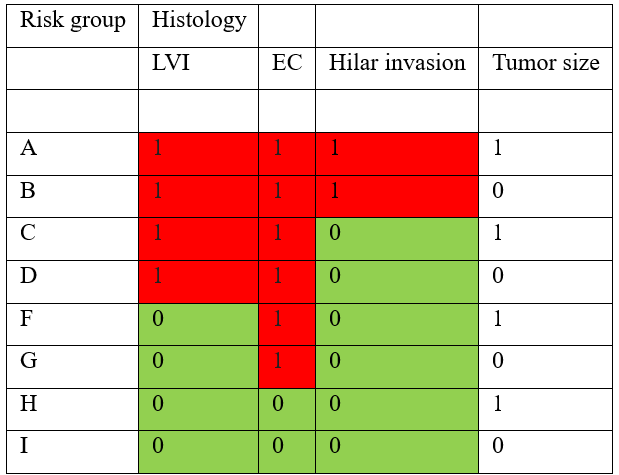
The table shows the risk groups of CS1 NSGCT with group A as the highest risk group and I as the lowest risk group. 0 shows an absent risk factor and 1 shows a group present risk factor. LVI = lymphovascular invasion.
References
- Wagner T, Toft BG, Lauritsen J, et al. Prognostic factors for relapse in patients with clinical stage I testicular non-seminoma: A nationwide, population-based cohort study. Eur J Cancer, 2024; 202: 114025.
- Wagner T, Toft BG, Lauritsen J, et al. Prognostic Factors for Relapse in Patients with Clinical Stage I Testicular Seminoma: A Nationwide, Population-Based Cohort Study. J Clin Oncol, 2024; 42: 81-89.
- Amin MB, Edge S, Green F, et al. AJCC Cancer Staging Manual, 8th ed. New York, Springer, 2017.
- Brierley JD, Gospodarowicz M, Witterkind C, eds. UICC TNM classification of malignant tumours. 8th ed. New York, Wiley Blackwell, 2016.
- Anon. International Germ Cell Consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol, 1997; 15.
- Incesu RB, Morra S, Scheipner L, et al. A population-based validation of the IGCCCG Update Consortium for survival in metastatic non-seminoma testis cancer. Jpn J Clin Oncol, 2024; 54: 592-598.
- Beyer J, Collette L, Sauve N, et al. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J Clin Oncol, 2021; 39: 1553-1562.
- Buhrer E, D'Haese D, Daugaard G, et al. Impact of teratoma on survival probabilities of patients with metastatic non-seminomatous germ cell cancer: Results from the IGCCCG Update Consortium. Eur J Cancer, 2024; 202: 114042.

