AF in HCM: Clinical and Paraclinical Predictive Factors, Impact and Therapeutic Means: About 21 Cases and Literature Review
Ouzzaouit S*, Laklalech S, Lamghari Y, Rhemimet C, Pr Fellat I and Pr Cherti M
Department of Cardiology, Ibn Sina Hospital, Rabat, Morocco
Received Date: 22/03/2025; Published Date: 02/05/2025
*Corresponding author: Sarra Ouzzaouit (MD), Department of Cardiology, Ibn Sina Hospital, Rabat, Morocco
Abstract
Background: The occurrence of AF during the course of HCM is common. The causes of its development are multifactorial, primarily related to anatomical, structural, and hemodynamic alterations specific to HCM, alongside additional genetic factors.
Objective: To understand the link between the two pathologies and to identify predictive factors for the occurrence of AF during HCM, in order to adapt appropriate therapeutic strategies, avoid complications, and reduce mortality.
Methods: Retrospective study of 21 cases of patients with HCM at the Cardiovascular B Unit of the Souissi Maternity Hospital between June 2013 and May 2023.
Results: Twenty-one patients were included, with a mean age of 51 ± 29 years. AF is the most common arrhythmia, with an incidence ranging from 12.5% to 24%. It is a prevalent cause of heart failure and stroke, hence the importance of screening, monitoring, and early treatment based on rhythm and heart rate control, as well as effective anticoagulation, including catheter radiofrequency ablation in some cases.
Conclusion: Atrial fibrillation is common in patients with hypertrophic cardiomyopathy due to structural, hemodynamic, and genetic mechanisms that promote left atrial remodeling, which worsens the prognosis and increases mortality. Regular monitoring and identification of predictive factors for AF are essential for adapting therapeutic strategies, including both drug and non-drug treatments. Anticoagulation is a key component of treatment to prevent thromboembolic complications and improve patient prognosis.
Keywords: Atrial fibrillation; Hypertrophic cardiomyopathy; Predictive factors; Thromboembolic complications
Introduction
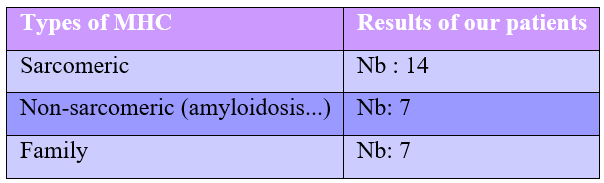
Hypertrophic Cardiomyopathy (HCM) is a group of heterogeneous cardiomyopathies of often genetic origin, accounting for 40% to 60% of HCM cases, due to a mutation in a gene encoding sarcomeric proteins, known as sarcomeric.
Sarcomeric HCM is transmitted in an autosomal dominant manner with heterogeneous penetrance, as are the phenotypic manifestations. It can be genetic or non-sarcomeric (metabolic diseases, neurodegenerative diseases, or infiltrative pathologies such as amyloidosis) or non-genetic with variable idiopathic causes. HCM is practically defined by an increase in LV wall thickness >15 mm (or >13 mm in a patient with a known HCM-positive relative or with a positive genetic test), in the absence of any abnormal loading conditions. AF is defined by the appearance of anarchic atrial activity characterized by rapid and totally disorganized depolarizations with disappearance of sinus rhythm and as a consequence the acceleration of the ventricular rate.
As a reminder, there are 3 types:
- paroxysmal AF, which corresponds to any episode of AF that appears and decreases spontaneously or by cardioversion in ≤ 7 days with restoration of the sinus rhythm, usually in less than 48 hours;
- Persistent AFib (lasts >7 days)
- Permanent AF which is an accepted AF
Atrial fibrillation is the most common arrhythmia during the course of the course in patients with HCM with a prevalence ranging from 12.5% to 24% and an annual incidence >2%/year [1,2], often poorly tolerated, and marks a pejorative turning point in the clinical history of HCM.
AF can be responsible for worsening heart failure, functional limitation, increased risk of thromboembolism and overall mortality.
Early diagnosis of supraventricular arrhythmia is an important issue for patients with HCM, as the risk of death in the presence of AF is multiplied by 4, and the thromboembolic risk is multiplied by 8, with an annual incidence of 3.75% [3-5].
In our work, we are interested in better understanding the pathophysiology linking the two pathologies, in knowing the impact of AF on HCM, and above all to study the risk factors and predictive factors for the occurrence of AF in patients with HCM.
AF is sometimes revealed by its embolic complications that are heavy in terms of morbidity and mortality, hence the importance of closer monitoring of these patients according to the available recommendations, for earlier therapeutic management and therefore fewer complications.
Therapeutic management is based on the control of ventricular rate and heart rate, mainly by beta-blockers and Amiodarone, and on long-term anticoagulation, which remains the cornerstone for preventing thromboembolic events (especially strokes).
Materials and Methods
This is a retrospective, descriptive study that includes all patients hospitalized in the Cardiology Department B CHU IBN SINA in Rabat with hypertrophic cardiomyopathy complicated by AF conducted over a period of 10 years, and which extends from June 2013 to May 2023, we were able to collect 21 patients diagnosed with MHC with AF out of 105 patients with MHC alone.
The objectives of our study are:
- Describe the current understanding of the pathophysiology of the association of AF with MHC
- Know the risk factors for the occurrence of AF during MHC
- Describe clinical and paraclinical predictors of AF during MHC
- To assess the impact of AF during MHC and its complications
- Calculate the risk of MSC according to ESC score
- Therapeutic interest: knowing the different therapeutic strategies
- The interest of anticoagulation which is independent of the CHA2DS2-vasc score
- Prognostic interest: reduce morbidity and mortality and improve the prognosis of patients associated with the disease through monitoring and early management
To this end, an operating sheet has been drawn up, including patients with MHC and AF who have already been hospitalized in the IBN Sina cardio B department in Rabat, men and women, over 15 years old, with clinical palpitations and functional limitation class II to IV of the NYHA, known to be carriers of MHC defined in echocardiography by a parietal thickness of the LV ≥ 15 mm (≥13 mm if family MHC ATCD), in the absence of any other cause that can lead to ventricular hypertrophy.
Exclusion criteria include the main differential diagnoses of HCM defined by the 2014 ESC recommendations, including subjects with tight aortic stenosis, aortic subvalvular anomaly increasing LV afterload, or severe or uncontrolled hypertension, also infantile forms are excluded (age <15 years)
As for the stratification of the risk of sudden death, it is calculated from a score developed by the ESC, it is not valid if the life expectancy is less than one year, in patients with non-sarcomeric HCM (infiltrative or metabolic), in competitive athletes and in children, or in the case of septal reduction, and must be reassessed at 5 years at the time of initial diagnosis and then every one to two years
Follow-up of patients is done on an outpatient basis including a clinical examination, a 12-lead ECG and a trans-thoracic examination every 1 to 2 years, except for patients with atrial dilation (the OG ≥45 mm), for whom this examination must be performed every 6 months, the same follow-up is recommended for the 24-hour ECG holter to detect asymptomatic arrhythmias, and if symptoms are present.
MRI evaluation may be considered every 5 years, or every 2 to 3 years in patients with active HCM
All pacemakers and defibrillators were interviewed by a senior rhythmologist, every 6 to 12 months or if there was a recent event.
Results
The study involved a number of 21 patients with MHC and AF, in whom this diagnosis was made at variable periods of initial diagnosis (MHC), spread over a period of 10 years
The mean age at diagnosis of HCM was 51 ± 29 years: the youngest patient was 32 years old and the oldest was 80 years old. These are 10 men (48%) and 11 women (52%). The sex ratio is 1.1 (F/M:1.1)
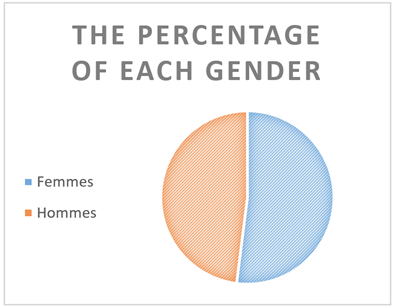
Figure 1: The percentage of each sex.
In terms of medical history in our series, 6 patients had a history of sudden death in the family and among them one patient had a heavy history of familial HCM, and was diagnosed with HCM through routine family screening and AF during a thromboembolic event (DALY):
- Sudden death of his mother at the age of 49
- Sudden death of his brother at the age of 30 known to be a carrier of HCM
- Sudden death of the 2 nephews with no known cause at a young age
Classical CDRDVs were found in our patients and dominated by smoking and hypertension
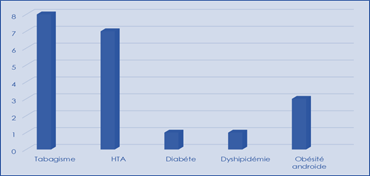
Figure 2: Distribution of FDRCvx.
Symptoms are found isolated or most often associated in each patient in our study and constitute the reason for initial consultation before hospitalization and whose cardiological exploration led to the diagnosis of MHC associated with AF:
- Dyspnea of exertional dyspnea stage II or III of NYHA (nb = 16; 76%)
- Palpitations with sudden onset and end on exertion and/or at rest (nb=14; 66%).
- Chest pain: often angina class II of SCC or precordialgia atypical on exercise or rest (nb=6; 28%)
- Episodes of lipothymia/syncope (nb: 5; 23%).
- Thromboembolic events (stroke) (nb: 4; 19%)
- A single patient who was asymptomatic, and not known to be a carrier of MHC and diagnosed after an episode of DALYs on AF (nb=1, 3%).
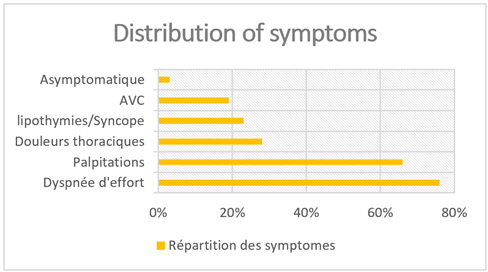
Figure 3: Symptoms found in MHC patients.
The characteristics of the electrocardiogram found in patients in our series are:
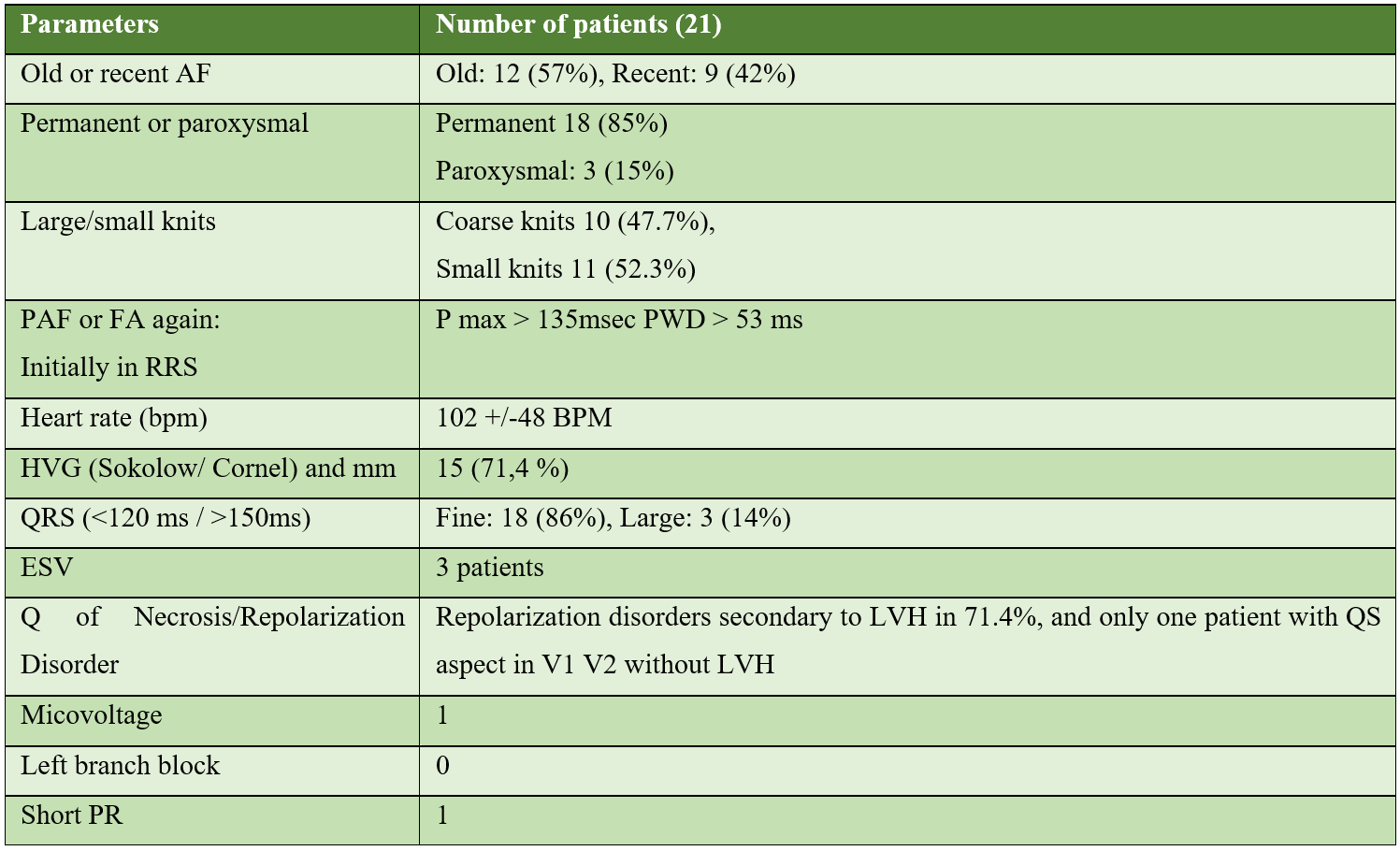
The 24-hour follow-up ECG holter performed in 10 patients in our study, i.e. 47%:
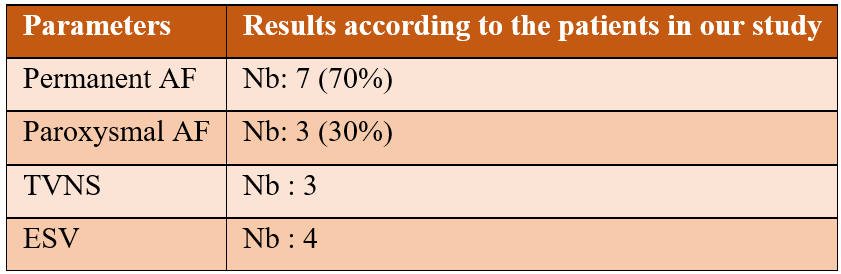
Patient-specific echocardiography data from our study:
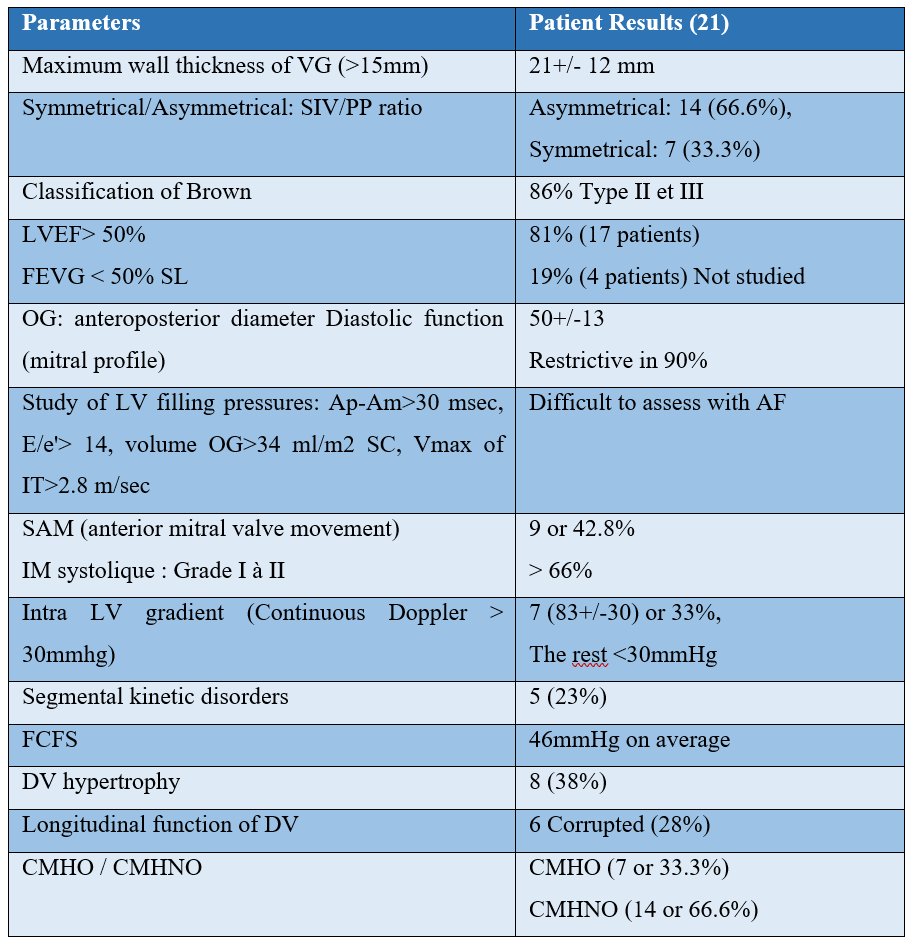
Cardiac MRI data: 10 of our patients who were able to benefit from an MRI, i.e. 47%:
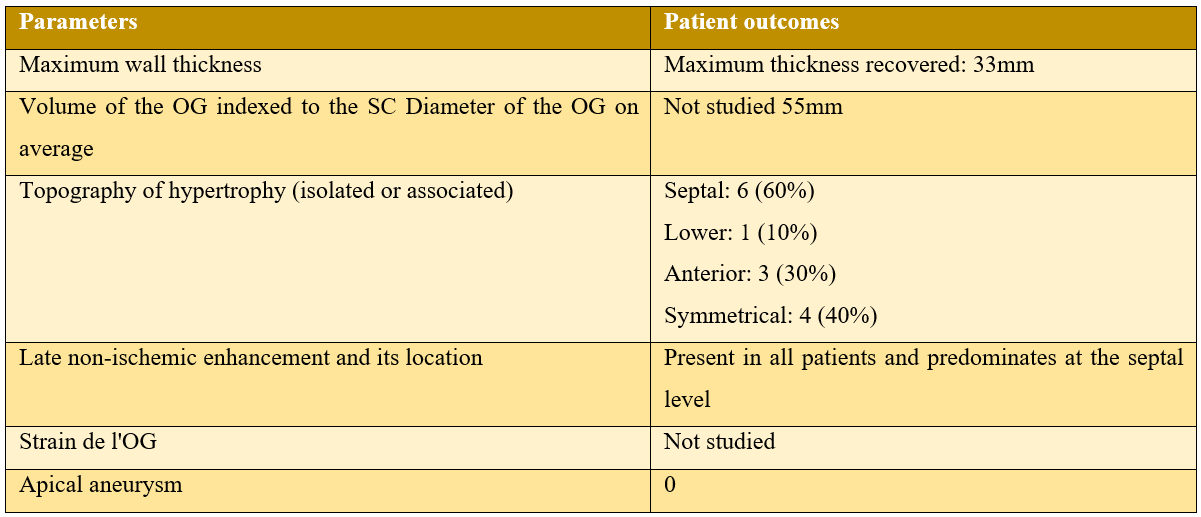
The etiologies of HCM in patients in our series:
As for the biological assessment:
- Troponin: was dosed in only 5 patients in our series who came back negative
- BNP and NT ProBNP: not measured in our patients
The risk score for sudden death in 5 years for HCM according to the ESC:
Among the criteria of the ESC SCD risk score are:
- age
- The TVNS (defined as > 3 consecutive ESVs with a frequency > 120 bpm)
- Maximum VG thickness
- The Family History of Sudden Death
- Unexplained syncope
- The anteroposterior diameter of the left atrium
- L’obstruction intra-VG maximale provocable au Valsalva
The risk is estimated to be low if <4%, intermediate if between 4 and 6% where the ICD should be discussed, or high if > 6% with indication for ICD placement
In primary prevention:
Assessing the risk of MS in our patients:
We excluded 6 of our patients from the calculation: 5 patients with symmetric non-sarcomeric MHC (suspected infiltrative) and another already implanted with an ICD
NB: one of our patients has already had septal alcohol to reduce his intra VG gradient and thus the obstruction.
This risk was broken down as follows:
- Low risk = 6 patients (42% mean)
- Intermediate risk = 6 patients (mean of 42%)
- High risk = 2 patients (an average of 14% of patients)
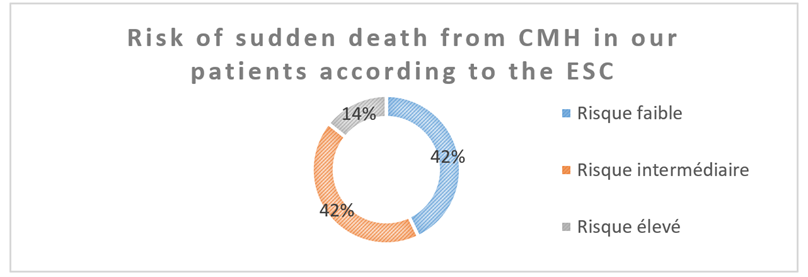
Figure 4: Risk of sudden death from HCM in our patients according to the ESC.
For secondary prevention:
In our series, the last patient excluded was already implanted with an ICD in front of a formal indication having a biventricular MHC at the dilated stage in LV dysfunction and who was doing SVTT in addition to his AF.
This risk assessment should be repeated every 1 to 2 years in at-risk patients who have not received an ICD.
Thromboembolic events revealing AF in our patients:
3 patients in our study presented a stroke revealing their AF, and one of them was diagnosed with HCM during this episode.
The care of our patients:
8 patients in our study with recent AF benefited from external electrical cardioversion (38%), after an ETO eliminating a contraindication, 7 which resulted in success with return to sinus rhythm.
Another serial patient had an OG thrombus on the ETO and a spontaneous contrast in the left atrium, thus contraindicating electrical cardioversion.
All our patients were put on anticoagulants: VKA (90%), DOACs (only one patient over 75 years old: 4%), a beta-blocker to maintain HR, and an antiarrhythmic (amiodarone) to maintain the sinus rhythm (see table below)
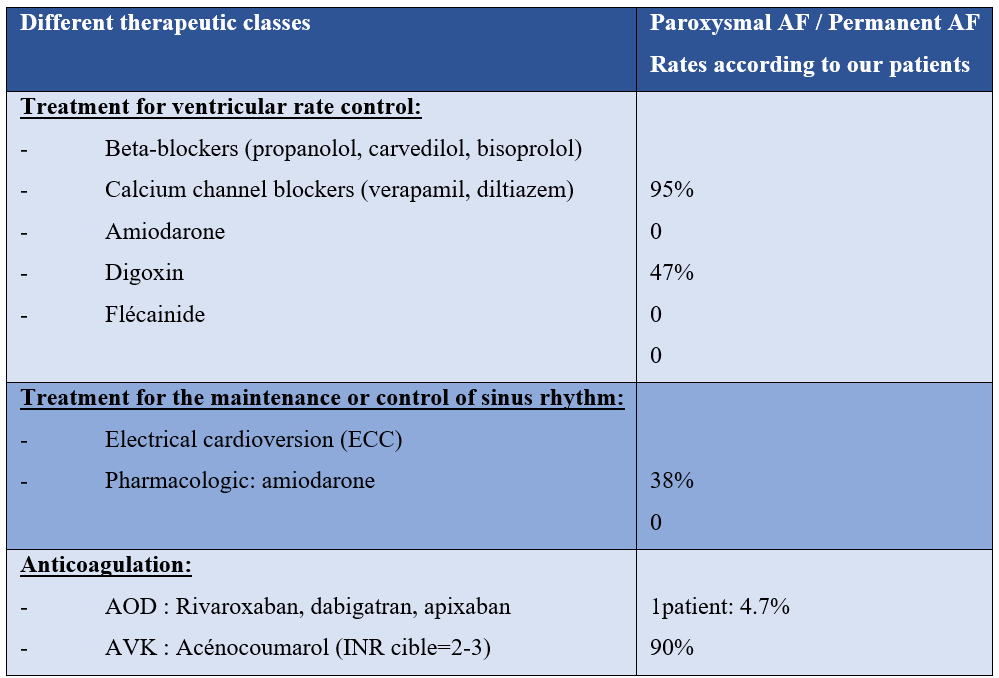
Follow-up of our patients:
- 2 patients who returned to sinus rhythm recurred their AF late (more than 3 months)
- A patient was implanted with a double-chamber pacemaker for sinus dysfunction and switched to AF to a 24-hour ECG holter.
- One of our patients was implanted with an implantable cardioverter defibrillator (ICD), as a secondary prevention (TVNS).
- 4 patients progressed to heart failure (systolic or diastolic) during follow-up, and were put on treatment for heart failure (diuretics, ACE inhibitors/ARA2, beta-blockers, sacubitril/valsartan, etc.) with great caution.
Limitations of the study:
The main limitation in our study was probably the non-detection of the various episodes of paroxysmal AF that go unnoticed, and which can be completely asymptomatic.
In addition, the strain, the indexed volume of OG has not been studied in most of our patients for different reasons, as well as cardiac MRI, only 10 of our patients have been able to benefit from it, i.e. 47% of patients.
Discussion
Definitions:
HCM is an inherited disease with autosomal dominant inheritance of variable penetrance, with different clinical phenotypes, characterized by hypertrophy mainly of the left ventricle by dysfunction of the sarcomeres due to genetic mutations encoding sarcomere proteins, the prevalence of this disease in the general population is estimated at 0.2% [6].
The ESC defined HCM according to its latest recommendations of 2014, as a group of heterogeneous cardiomyopathies characterized by structural and functional abnormalities of the ventricular myocardium that are not explained by abnormalities of loading conditions (in the absence of severe hypertension, aortic stenosis or other abnormality increasing afterload) or by coronary artery disease limiting blood flow.
Defined practically on imaging (either ETT, MRI or CT) by the presence of an increase in the parietal thickness of the LV in tele-diastole ≥ 15 mm of at least one segment of the myocardium, or ≥13 mm in patients with relatives with HCM or with a positive genetic test, with an undilated LV and in the absence of abnormalities in the loading conditions (absence of another cause of hypertrophy).
Vulpian took the first step in the development of the diagnosis of HCM by identifying it anatomically in a patient in 1868 [7], HCM can be either primary; where the heart is the only organ involved, or secondary associating extra-cardiac manifestations that are part of the systemic disorder, they are then classified into genetic/familial and non-genetic/non-familial.
Atrial fibrillation (AF) is the most common arrhythmia in patients with HCM [8,9], mainly due to the process of dilation, remodeling and fibrosis of the left atrium in addition to diastolic dysfunction, its presence usually leads to progressive functional deterioration, an increase in the frequency of hospitalizations for heart failure and an increased thromboembolic risk with increased rates of Overall mortality [10].
Thus, patients with MHC have a 4 to 6 times higher probability of developing AF compared to the general population [11,12].
Prevalence of AF in HCM:
According to statistics, 1 in 500 people is affected by HCM, with a large percentage of undiagnosed patients, and its prevalence is higher in the African population and mainly seen in younger men [12].
The reported prevalence of AF in HCM ranges from 12.5% to 24% with an annual incidence >2%/year [14,15], up to 40% in elderly patients with HCM over 70 years of age.
A meta-analysis of 7381 patients reported an overall incidence of AF of 3.08% per 100 patients per year and a lifetime prevalence of 22.5% [51], AF is a progressive arrhythmia in patients with HCM with major clinical impact, and tends to be paroxysmal in two-thirds of patients, while the rest have persistent/permanent AF [16,17].
The most important published studies describing the prevalence and incidence of AF in patients with MHC are summarized in the following table:
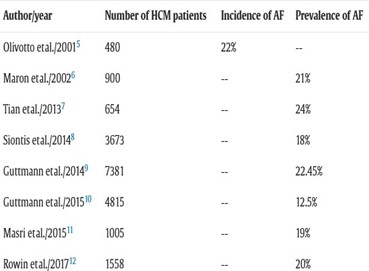
Figure 5: The prevalence or incidence of AF in patients with HCM according to the American ACC/AHA recommendations 202.
Clinically silent episodes of AF account for approximately 24% in patients with MHC with implanted defibrillators [18], it is more common in older patients and in patients with LV ejection tract obstruction [14,19]
In our study of 105 patients with HCM, 21 who were diagnosed with AF or 20%, over a period of 10 years, 3 of our patients had paroxysmal AF revealed during follow-up by the 24-hour ECG holter, with only one passage to permanent AF during follow-up, and 4 patients between them in AF were revealed by a thromboembolic event (DALY), one of which was the initial manifestation revealing his HCM.
Risk factors that increase the incidence of AF in HCM:
According to the 2020 ESC recommendations, general factors were implicated in the increase in the incidence of AF (Figure 6).
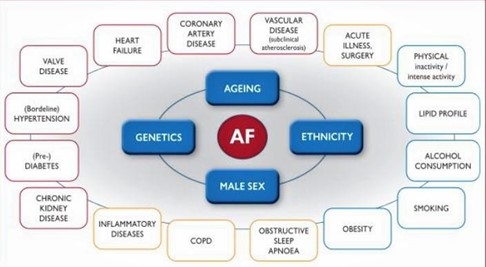
Figure 6: Risk factors involved in increasing the incidence of AF according to the 2020 ESC recommendations.
Several independent risk factors and predictors of the onset of AF in patients with MHC have been described, such as size, function of the left atrium, diameter, and especially the volume of the OG as well as the deformity of the free wall of the left atrium (the strain); all of these factors seem to be consistently correlated with the onset of AF in patients with MHC [14,20-22].
Clinical factors, such as age, female gender, NYHA (New York Heart Association) class, hypertension, and vascular disease, have also been associated with the onset of AF [23].
The most powerful parameters recognized according to studies and associated with AF are: age, hypertension, diameter and atriol volumes, also a fraction of left ventricular ejection lowered by echocardiography or magnetic resonance imaging (MRI):
Terrain:
- Age is considered to be one of the most dominant predictors of the occurrence of AF in patients with MHC [24], according to several cohort studies, HFC-associated AF rarely develops in patients under 30 years of age and it is more common in older age groups [25]. Age cut-offs ranging from 40 to 50 years, have been shown to be vital indicators of AF development in the general population and in patients with HCM specifically [25].
In our study, the average age of our patients with MHC with AF was 51 years, the rate of AF occurrence increased gradually with age, beyond 50 years of age, there were 13 patients, compared to 8 patients aged <50 years, but no patients under 30 years of age were found, which is consistent with previous studies.
- Two large studies report a greater incidence of AF in HCM in women with an incidence of 43% in the first study [26], and 39% in the multicenter study[27]
- The results are consistent with our study: 52% of MHC patients with AF were women.
- Functional limitation: dyspnea, HF, obstruction
Dyspnea is a symptom often found in patients with HCM, which worsens during the course of the disease, related to diastolic dysfunction, especially in the presence of an intra-LV gradient, heart failure or with the appearance of AF
Some patients with de novo AF may be asymptomatic, and others with stage III and VI NYHA in case of congestion
A study conducted by Maron MS et al, on 320 patients with obstructive HCM associated with AF, 225 of them had intra LV gradients with subaortic obstruction and experienced heart failure symptoms detectable only on exertion [28] in fact, diastolic dysfunction is considered the main cause of heart failure in patients with MHC [29].
In the patients in our series, dyspnea was the master of symptoms representing 76% of cases, followed by the rest of the symptoms: palpitations (66.6%), angina (28%), lipothymia (23%).
In our series, there were 7 patients with obstructive MHC with AF, and intra-LV gradient, who appeared to be more symptomatic than the rest without obstruction, 4 of whom had repetitive diastolic heart failure flare-ups, which is consistent with previous studies.
Sinus P wave and AF incidence:
A study of 80 patients reported that the duration of the Pmax wave >134.5 ms and a Pwd wave dispersion value > 52.5 ms distinguished patients who developed AFib from controls with a sensitivity of 96% and a specificity of 91% [30].
NB: Pmax reflects the prolongation of the duration of intra- and inter-atrial conduction
PWD: testifies to the heterogeneity of atrial conduction by measuring the difference between the longest and shortest duration (Pmax-PWD)
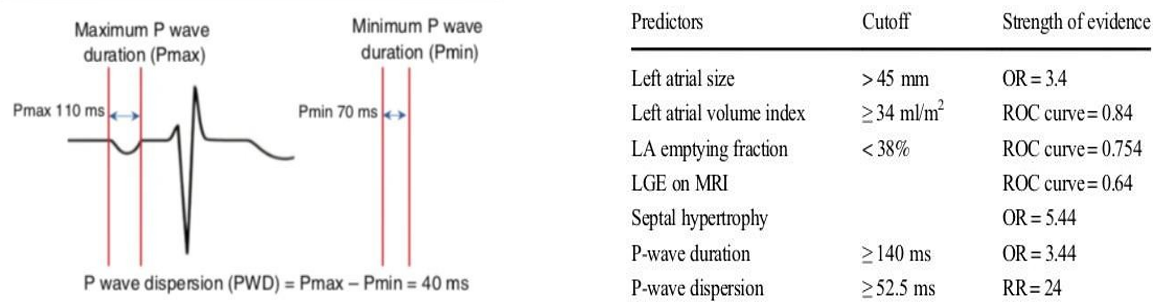
Figure 7: Measurement of P max and PWD on ECG Figure 8: Predictors of AF in patients with HCM.
In our study series: half of our patients followed for MHC and who later developed permanent AF had a maximum duration of the P> wave 135 msec and a PWD> 53msec measured in sinus rhythm before the onset of AF.
Evaluation of the left atrium by cardiac imaging:
Is based on several methods, including transthoracic echocardiography and MRI, analyzing morphological and functional criteria.
Regarding ETT, various studies have determined predictive thresholds for the risk of atrial fibrillation in patients with hypertrophic cardiomyopathy, including an anterior-posterior OG diameter > 45 mm and an indexed volume > 34 ml/m² [31].
The measurement of the volume of the OG, carried out by the disc summation method (Simpson biplane) on 4 and 2 chamber sections and indexed to the body surface area, offers a more accurate and reproducible estimate, comparable to the values obtained by MRI and cardiac CT scan.
In a study conducted by Tani T et al. on 141 patients with HCM, shows a linear relationship between the size of the OG volume and the incidence of paroxysmal AF (Figure 9).
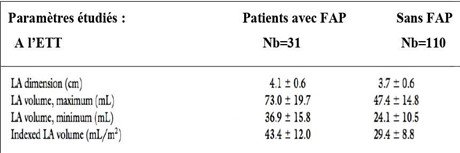
Figure 9: Comparative measurements of OG diameter and volume in MHC patients with FAP and MHC without FAP.
Indeed, the OG of patients with paroxysmal AF and MHC tends to have a larger volume, lower ejection fraction, and lower values of the overall longitudinal deformation peak [32,33] and also have a greater amount of fibrosis, as indicated by the percentage of late gadolinium enhancement on MRI (Figure 10).
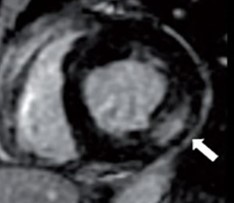
Figure 10: MRI image showing late intramyocardial enhancement in the basal segment of the inferolateral wall.
Thus, an OG volume >40 ml/m2 is linked to the risk of developing paroxysmal AF with a sensitivity and specificity of 80% and 73% respectively [34].
In another study of 427 patients with MHC, a final diastolic volume of OG > 118 mL and a emptying fraction of OG < 38%, predicted a risk of paroxysmal AF in patients with MHC [35].
In our series, the measurement of OG volume was performed only in some patients, all with an OG volume >40ml/m²
The anteroposterior diameter of the OG:
In a meta-analysis conducted by Guttmann OP et al. in 2014 involving 7381 patients (33 studies) found that the two predictors of the occurrence of AF were: age and anterior-posterior diameter of the OG: the diameter of the OG was 38 mm in patients with sinus rhythm HCM compared to 45 mm for those with chronic AF at the time of initial diagnosis [31].
In another study of 480 patients with Olivotto (age at diagnosis> 50 years) in 2001, with a nine-year follow-up, an OG diameter > 45 mm was significantly associated with a higher risk of AF: 107/480 patients who developed AF as a complication of the disease [25].
In our study of 21 patients, the mean anteroposterior diameter was 50 mm, with a maximum diameter of 65 mm and a minimum of 42 mm, and these measurements are consistent with the data in the literature.
3 of our patients had paroxysmal AF, and they are all women, with an average AP diameter calculated at 49mm.
Fonction de l'OG :
The atrial function can be summarized in three functions: reservoir, conduit and pump, several methods to study it, especially the strain of the OG.
And since there are limitations in any study: the OG Function was not studied in our series for various reasons
Contribution of MRI in the study of OG:
Several studies have concluded that the two most specific parameters in the prediction of AF are: the increase in the volume of the OG and the alteration of its function
According to several studies, the location of RTs and therefore fibrosis deposition at the posterior insertion site of the RV/LV independently predicts the onset of AF de novo, or screening patients predisposed to developing AF.
In our series, late enhancement testifying to fibrosis was found in all our patients who had an MRI, i.e. 47%, with a predominant topographic location in a septal (Septale: 60%; Anterior: 30%; Lower: 10%), and an average diameter of 55mm
Septal hypertrophy and obstruction:
Siontis KC et al. conducted a retrospective analysis of more than 3,500 patients with HCM between 1975 and 2012 and concluded that several echocardiographic markers in patients with HCM, such as left ventricular wall thickness and left ventricular stiffness, are associated with a higher risk of AF [36].
Indeed, a study of 1,360 patients with HCM concluded that septal hypertrophy on ETT and/or extensive MRI is associated with a higher risk of AF [37].
In our patients in the series, septal hypertrophy was predominant, with a mean thickness at 21mm and a maximum thickness at 33mm
Le score HCM –AF:
The HCM-AF score is a novel validated predictive tool to identify AF risk in MHC, it reliably stratifies patients with MHC based on AF risk, this score was developed in a large diverse cohort of patients assessed at Tufts HCM Center and provides individualized AF risk estimates at 2 years and 5 years using 4 parameters: the diameter of the OG, the age at initial diagnosis of HCM, the CHADSVASC score, and the presence of heart failure symptoms.
The HCM-AF score stratifies the risk into low (<1.0%/year; score ≤17), intermediate (1.0-2.0%/year; score 18-21) and high (>2.0%/year; score ≥22) for the development of AF for patients The identification of these MHC patients at higher risk of AF allows for early management and appropriate management during the clinical course
In our series, we didn't need to calculate this score, since all our patients were in AF.
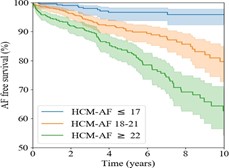
Figure 11: Survival stratified by HCM-AF score.
Ischemia and AF in HCM
According to a recent study in August 2020 that investigated the impact of ischemia on the occurrence of AF in HCM and concluded that there is a clear association between coronary microvascular dysfunction and AF genesis and that a 10% worsening of ischemia is accompanied by a 67% increased risk of developing AF de novo, and that this association is completely independent of all known risk factors predictive of AF in HCM [38].
Complications of AFib in HCM:
AF is an independent predictor of mortality in patients with HCM, and is associated with a risk of death up to four times higher than sinus rhythm.
The majority of deaths in the AF group were related to thromboembolic events (mainly stroke) and worsening heart failure, there are some cases of sudden sudden cardiac death due to deterioration of AF to ventricular tachycardia, especially in the presence of pre-excitement.
Embolics:
In a large meta-analysis of 7381 patients, the incidence of thromboembolic events was 3.8% per year and the overall prevalence was 27.1% [27].
In another study of 480 patients, spread over a period of 9 years, AF was identified as an independent factor responsible for the increased morbidity and mortality rates in MHC patients and that ischemic stroke was eight times more common in the AF group (21%) than in the sinus rhythm HCM group (2.6%) [25], and showed that the thromboembolic risk in AF is not related to the type of AF (paroxysmal or persistent), in fact, the ET risk in MHC patients who experienced a single episode of paroxysmal AF was identical to those who experienced many episodes.
Another recent study that followed MHC patients in order to detect embolic events, revealed a significant prevalence of unknown AF for 57.4% of ET accidents [25], hence the interest of screening in follow-up thanks to repeated ECG holter recordings in patients at risk.
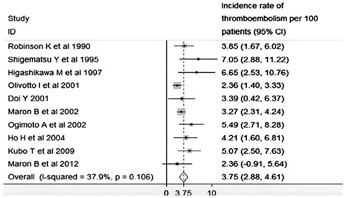
Figure 11: Incidence of AF ET accidents in HCM according to different studies.
In our case series, there were 3 patients who had a stroke on a recent AF, and were put on anticoagulants at a distance, with INRs that were within the range (target INR between 2 and 3)
Heart failure:
The natural history of progression of MHC is towards heart failure, the appearance of AF during MHC has an impact first clinically, then on ventricular function but also on the patient's prognosis.
According to a study, about one in seven patients had symptoms of heart failure, mediated in 64% of cases by atrial arrhythmia [39], but also by a progression to the restrictive form of MHC which is due to reverse remodeling, leading to ventricular dilation and thinning of the walls, it would occur according to Harris et al in 3.6% of MHC cases, most often concomitantly with atrial fibrillation and in young patients and which has a very poor prognosis with an annual mortality of 11% [40].
According to the Olivotto and Maron study conducted on 480 MHC patients, 22% of patients had AF at different periods: either at the time of initial diagnosis or during the course of the disease and showed that the onset of de novo AF led to HF symptoms in 84% of these patients (OAP, dyspnea, IMO ...) and did not result in HF symptoms in the remaining minority (16%), as revealed during routine routine examinations in MHC patients.
In this group of patients, AF was responsible for the long-term deterioration of the functional class of the NYHA: 95% of patients with AF progressed to dyspnea stage III and IV of the NYHA compared to the rest of the patients in sinus rhythm.
About our study, we had 6 patients at the HF stage, i.e. 28% of the total number of our patients. The mean age of onset of decompensation relapses was 66 years, and the relapse occurs at a distance, years after the date of HCM discovery, with an average of 6 years in men, and 7 years in women.
All of our MHC patients with AF at the heart failure stage had functional limitation with progressive worsening of their dyspnea, and frequent hospitalizations for HF decompensation flare-ups, which is consistent with previous studies.
Sudden death:
Atrial fibrillation is an independent factor in the increase in mortality and morbidity in this pathology, the risk of sudden death described is low and generally due to the deterioration of AF in VT and was significantly higher in patients with flow obstruction or early development of AF (≤50 years) or with pre-arousal.
Therapeutic measures:
Drug treatment:
HR Control:
- Beta-blockers : negative inotropes, by lowering contractility, reduce symptoms related to obstruction (dyspnea and chest pain) and improve exercise tolerance, mainly by lowering heart rate and lengthening diastole (chronotropic negative)
- Calcium channel blockers are the cornerstone of AV node suppression therapy and have been used for a long duration: A study was performed by Hanrath in 11 patients with MHC treated with a calcium channel blocker (Verapamil), the results revealed a significant improvement in relaxation and filling of the left ventricle, 10 minutes after intravenous injection of Verapamil, and an extension of the left ventricular iso-volume relaxation time.
- Another study done by Roberts on 115 patients with MHC and AF treated with Digoxin, and in whom the ventricular rate was fast (>120 beats/minute), the time it took to control the rate was 11.6 hours to bring the heart rate down to <100 BPM, the study concluded that the fast ventricular rate is inconsistent and ineffective [29]. However, the use of Digoxin is limited by the unpredictability of its adverse effects such as arrhythmias and gastrointestinal symptoms.
In the patients in our series, 95% were put on beta-blockers with a good clinical course. None of our patients have been put on IC or Digoxin.
Heart rate control:
In a large randomized trial carried out by Al-Khatib SM on 28,836 patients with HCM, the study revealed that there is an improvement in the symptoms of these patients with a significant decrease in mortality after the use of antiarrhythmics.
In a study of 52 MHC patients, performed by Malasana, 29 patients experienced a rhythm disorder including 24 AF, and were brought back to a sinus rhythm by Amiodarone which allowed a sinus rhythm to be maintained in 22 of the 29 patients after a five-year follow-up period, the results concluded that after treatment with amiodarone, These patients had experienced a small number of embolic episodes [41].
In a retrospective analysis performed by Robinson in 174 patients with HCM and AF who were followed for 11 years, 25 of 52 patients were treated with conventional therapy alone, and seven with amiodarone alone, and the remaining 20 patients received standard therapy first, the study concluded a significant decrease in episodic recurrence of ET, improved quality of life and decreased incidence of AF by maintaining sinus rhythm with amiodarone [42] (Figure 12).
One study concluded that Sotalol significantly improves exercise tolerance and effectively suppresses supraventricular and ventricular arrhythmias in patients with HCM, especially young ones [43].
Dofetilis is a class III antiarrhythmic, a study performed by Moore JC on 1404 patients with MHC concluded that Dofetilis was well tolerated in MHC patients in AF and was able to manage AF in 21/25 (84%) cases [44].
In the patients in our study, the only antiarrhythmic drug used was amiodarone in 47% of cases.
Prophylaxie thromboembolique :
Alternatives to Warfarin include non-vitamin K antagonist oral anticoagulants (NOACs), in fact, a cohort study was performed by Jung H et al. on 2458 Korean patients with MHC that compared two groups: a group of Warfarin-treated patients with MHC and AF (n=955), and a group of patients treated with DOACs (n=1504), the study concluded that the risk of stroke and major bleeding was similar in patients with HCM and AF treated with DOACs as with Warfarin, however, all cardiovascular and mortality events were lower compared to those taking Warfarin, suggesting that patients with MHC and AF can be safely and effectively treated with DOACs [45] (Figure 12).
In a retrospective analysis of 4821 patients with HCM, 9.8% of subjects with a CHA2DS2-VASc score of 0 experienced a thromboembolic event during the 10 years of follow-up [27], this study found that advanced age, presence of AF, left ventricular wall thickness, history of thromboembolism, advanced NYHA class, increased OG volume were statistically significant predictors of increased risk of thromboembolism, while the use of vitamin K antagonists (VKAs) was associated with a 54.8% relative risk.
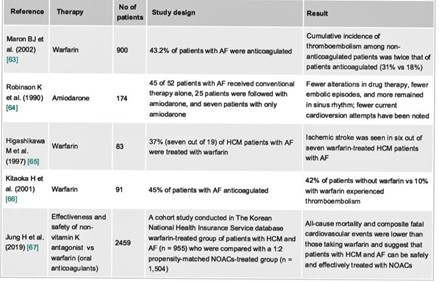
Figure 12: Thromboembolic prophylaxis of AF in HCM: summary of different studies conducted between 1990 and 2019.
All our patients were systematically put on anticoagulants: VKA (90%) with INR in the therapeutic range (target between 2 and 3), or on DOACs (4%, only one patient over 75 years old)
Radiofrequency AFib ablation:
A study performed by Di Donna P et al. on 61 patients with HCM with paroxysmal or long-term AF, who received radiofrequency catheter ablation (RFCA), (by isolation of the pulmonary vein), 41 patients regained a sinus rhythm with improvement in symptoms, the study concluded a significant decrease in AF thanks to RFCA in patients with MHC [45].
Another clinical study performed by Walters in which 83 patients with AF under the age of 75 were followed for 12 months having undergone early ablative catheter intervention and, after the procedure, they were followed with detailed evaluation by their treating physicians, the study concluded a significant improvement in reverse structural remodeling in these patients and an improvement in their quality of life with decrease of recurrence of AF [46].
In AF recurrence, the posterior wall of the left atrium plays a key role, but the benefits of isolating the posterior wall of the left atrium are unclear.
A meta-analysis performed on 594 patients with AF and compared posterior wall ablation with other ablation groups, those in the posterior wall had a lower rate of recurrence of atrial arrhythmia, the study concluded a significant decrease in AF recurrence without any procedure-related complications [47].
Several studies have shown that lifestyle modifications, such as a healthy diet and physical activity, may reduce the incidence of AFib and allow for successful ablations as well as the treatment of underlying comorbidities such as diabetes, hypertension, and sleep apnea.
None of our patients had their AF ablated by radiofrequency, but one of our patients benefited from septal alcohol to reduce intra-LV obstruction.
Follow-up of hcm patients with afib:
Patients with HCM, regardless of the context of AF, should be followed for life for evaluation and detection of symptom onset and/or change
The frequency of these medical check-ups should be chosen according to the patient's symptoms, age and severity of illness [48] :
- In general, for asymptomatic patients: outpatient cardiological follow-up including clinical examination, 12-lead ECG and transthoracic examination is required; should be performed every 1-2 years, except for patients with atrial dilation (the OG ≥45 mm), for whom this examination should be performed every 6 months, the same follow-up is recommended for the 24/48-hour ECG to detect unnoticed atrial or ventricular arrhythmias asymptomatic, and in case of symptoms: palpitations or syncopes (I, C).
- MRI evaluation may be considered every 5 years, or every 2 to 3 years in patients with active MHC (IIb, C).
- It is recommended that the MS risk score be calculated at 5 years at initial diagnosis and should be reassessed every one to two years or whenever there is a change in clinical status (I, B).
In clinical practice, it is recommended to perform an ECG approximately 2 to 4 weeks after the first intake of an antiarrhythmic drug: This control should aim to exclude brady arrhythmias and QT prolongation, requiring discontinuation or reduction of the dose if the QTc interval exceeds 480 milliseconds during the initial administration and especially concomitantly with other QT-prolonging drugs should be avoided.
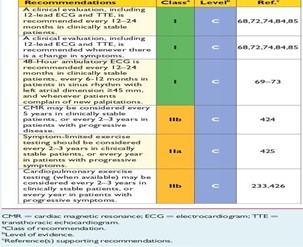
Figure 13: ESC 2014 recommendations for the follow-up of MHC patients.
The follow-up of our patients was done according to the recommendations of the ESC already mentioned (as far as possible), a clinical follow-up (symptoms at rest and during exercise, tolerance of medications, side effects...), and paraclinical follow-up (ECG, HOLTER ECG, ETT, biological assessment if necessary...) which allows systematic screening of our patients for the occurrence of complications and at the same time a good management.
Conclusion
The appearance of AF during the evolution of HCM is frequent with an incidence of 22% and annual at more than 2%/year, the causes of its development are multifactorial, mainly related to structural and hemodynamic anatomical alterations specific to HCM alongside added genetic factors; and then related to processes of mechanical and electrical remodeling of the usual left atrium in AFib, especially due to the progressive dilation of the OG, this association leads to a higher mortality rate and a poor prognosis
Thromboembolic complications may be the mode of detection of AF, hence regular monitoring is necessary in patients with MHC not yet known in AF, so it is necessary to know the predictive factors of AF occurrence in order to adapt appropriate therapeutic strategies as well as clinical and paraclinical monitoring.
Several pharmacological and non-pharmacological strategies are available for the treatment of acute AF, AF control, and prevention of recurrence; Beta-blockers and amiodarone, as well as catheter radiofrequency ablation in some cases, are options to keep patients in sinus rhythm, treatment protocols should be tailored to each patient
Anticoagulation is the cornerstone of the treatment of patients with HCM from the first labeled episode of AF, anticoagulation with Warfarin reduces thrombus formation and thus decreases the occurrence of cerebrovascular thrombotic events, DOACs are an alternative, and it is the pillar of the fight against mortality given the complications
this study has allowed us to better understand the pathophysiology that links HCM and AF, and its impact on the evolution of the disease, and especially the predictive factors of its occurrence requiring clinical and paraclinical monitoring and whose rhythm depends on the profile of each patient; Hoping that this study will be an initiation for several studies on larger series, in order to interact early, optimize treatment, improve the symptoms and quality of life of patients and thus the prognosis of the disease.
Author contribution:
MB: Study concept, Data collection, Data analysis, writing the paper.
RL: Study concept, Data collection, Data analysis.
RF: Study concept, Data analysis, writing the paper.
NM: Supervision and data validation
IA: Supervision and data validation
AB: Supervision and data validation
All authors reviewed the final manuscript.
Acknowledgments: Not applicable.
Funding Sources: There are no funding sources to declare.
Ethics approval and consent to participate: Not applicable.
Competing interests: None.
Provenance and peer review: Not commissioned, externally peer-reviewed
Consent: The authors confirm that they have provided written consent for the submission and publication of this case. The authors confirm that their written consent for the submission and publication of this case complies with the Committee on Publication Ethics (COPE) guidelines.
Availability of data and materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
References
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 2001; 104(21): 2517e2524.
- Rowin Ethan J, Hausvater Anais, Link Mark S, et al. Clinical Profile and Con- sequences of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation, 2017; 136(25): 2420e2436. https://doi.org/10.1161/CIRCULATIONAHA.117.029267.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH and Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 2001; 104: 2517-2524.
- Tian T, Wang Y, Sun K, Wang J, Zou Y, Zhang W, et al. Clinical profile and prognostic significance of atrial fibrillation in hypertrophic cardiomyopathy. Cardiology, 2013; 126: 258-264.
- Guttmann OP, Rahman MS, O’Mahony C, Anas tasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart, 2014; 100: 465-472.
- Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med, 1997; 336: 775–785.
- REview I. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations,diagnosis, and therapy. Circ Res, 2017; 121: 749-770. 10.1161/CIRCRESAHA.117.311059.
- Vaidya K, Semsarian C, Chan KH. Atrial fibrillation in hypertrophic cardiomyopathy. Heart Lung Circ, 2017; 26: 975-982.
- El-Battrawy I, Borggrefe M, Akin I. Atrial fibrillation in hypertrophic cardiomyopathy. JACC Heart Fail 2018; 6: 807. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace, 2016; 18(11): 1609e1678. https://doi.org/10.1093/europace/euw295.
- Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation, 2000; 102: 858-864.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace, 2016; 18(11): 1609e1678. https://doi.org/10.1093/europace/euw295.
- Lee SE, Park JK, Uhm JS, et al. Impact of atrial fibrillation on the clinical course of apical hypertrophic cardiomyopathy. Heart, 2017; 103(19): 1496e1501. https://doi.org/10.1136/heartjnl-2016-310720.
- Eberly LA, Day SM, Ashley EA, et al. Association of race with disease expression and clinical outcomes among patients with hypertrophic cardiomyopathy. JAMA Cardiol, 2020; 5: 83-91. 10.1001/jamacardio.2019.4638.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 2001; 104(21): 2517e2524.
- Rowin Ethan J, Hausvater Anais, Link Mark S, et al. Clinical Profile and Con- sequences of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Circulation, 2017; 136(25): 2420e2436. https://doi.org/10.1161/CIRCULATIONAHA.117.029267.
- Rowin Ethan J, Alexander Orfanos, Mark Estes NA, et al. Occurrence and Natural History of Clinically Silent Episodes of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Am J Cardiol, 2017; 119(11): 1862e1865. https://doi.org/10.1016/j.amjcard.2017.02.040.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 2001; 104: 2517-2524.
- Rowin Ethan J, Alexander Orfanos, Mark Estes NA, et al. Occurrence and Natural History of Clinically Silent Episodes of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Am J Cardiol, 2017; 119(11): 1862e1865. https://doi.org/10.1016/j.amjcard.2017.02.040.
- van Velzen HG, Theuns DA, Yap SC, Michels M, Schinkel AF. Incidence of device- detected atrial fibrillation and long-term outcomes in patients with hypertrophic cardiomyopathy. Am J Cardiol, 2017; 119: 100-105.
- Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart, 2014; 100(6): 465e472.
- Debonnaire Philippe, Joyce Emer, Hiemstra Yasmine, et al. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ Arrhythm Electrophysiol, 2017; 10(2): e004052. https://doi.org/10.1161/CIRCEP.116.004052.
- Losi MA, Betocchi S, Aversa M, et al. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol, 2004; 94: 895e900. https://doi.org/10.1016/j.amjcard.2004.06.024.
- Guttmann Oliver P, Pavlou Menelaos, O'Mahony Constantinos, et al. Hypertrophic Cardiomyopathy Outcomes Investigators. Predictors of atrial fibrillation in hypertrophic cardiomyopathy. Heart, 2017; 103(9): 672e678. https://doi.org/10.1136/heartjnl-2016- 309672.
- Elliott PM, Anastasakis A, Borger Michael A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J, 2014; 35(39): 2733e2779. https://doi.org/10.1093/eurheartj/ehu284.
- Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation, 2001; 104: 2517-2524. 10.1161/hc4601.097997.
- Iacopo Olivotto, Franco Cecchi, Susan A Casey RN, Alberto Dolara, Jay H Traverse, Barry J Maron. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy, 104: 2517-2524.
- Guttmann OP, Pavlou M, O’Mahony C, et al. Heart 2016, Predictors of atrial fibrillation in hypertrophic cardiomyopathy, 2016. doi:10.1136/ heartjnl-2016-309672.
- Braunwald E, Ebert PA. Hemodynamic alterations in idiopathic hypertrophic subaortic stenosis induced by sympathomimetic drugs. Am J Cardiol, 1962; 10: 489-495. 10.1016/0002- 9149(62)90373-9.
- Guttmann OP, Pavlou M, O'Mahony C, et al. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail, 2015; 17: 837-845. 10.1002/ejhf.316.
- Liouville H. Sub-aortic cardiac stricture. Gaz Med., Paris, 1869; 24: 161.
- Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart, 2014; 100(6): 465–472.
- Sivalokanathan Sanjay, Zghaib Tarek, V Greenland Gabriela, et al. Hypertrophic Cardiomyopathy Patients With Paroxysmal Atrial Fibrillation Have a High Burden of Left Atrial Fibrosis by Cardiac Magnetic Resonance Imaging. JACC Clin Electrophysiol, 2019; 5(3): 364e375. https://doi.org/10.1016/jacep.2018.10.016.
- Tayal B, Malahfji M, Buergler JM, Shah DJ, Nagueh SF. Hemodynamic determinants of left atrial strain in patients with hypertrophic cardiomyopathy: A combined echocardiography and CMR study. PLoS One, 2021; 16: e0245934.
- Tani T, Tanabe K, Ono M, et al. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr, 2004; 17: 644-648. 10.1016/j.echo.2004.02.010.
- Maron BJ, Haas TS, Maron MS, Lesser JR, Browning JA, Chan RH, et al. Left atrial re modeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am J Cardiol, 2014; 113(8): 1394–1400.
- Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc, 2014; 3: e001002. doi: 10.1161/JAHA.114.001002.
- Park KM, Im SI, Kim EK, Lee SC, Park SJ, Kim JS, et al. Atrial fibrillation in hypertrophic cardiomyopathy: is the extent of septal hypertrophy important PLoS One, 2016; 11(6): e0156410.
- Aguiar Rosa S, Thomas B, Fiarresga A, Papoila AL, Alves M, Pereira R, et al. The Impact of Ischemia Assessed by Magnetic Resonance on Functional, Arrhythmic, and Imaging Features of Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med, 2021; 8: 761860. doi: 10.3389/fcvm.2021.761860.
- Guttmann OP, Pavlou M, O’Mahony C, Monserrat L, Anastasakis A, Rapezzi C, et al. Hypertrophic Cardiomyopathy Outcomes Investigators. Prediction of thrombo-embolic risk in patients with hypertrophic cardiomyopathy (HCM Risk-CVA). Eur J Heart Fail, 2015; 17: 837-845.
- Harris KM, et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation, 2006; 114: 216– 225.
- Malasana G, Day JD, Bunch TJ. Atrial fibrillation in hypertrophic obstructive cardiomyopathy antiarrhythmics, ablation and more!. J Atr Fibrillation, 2009; 2: 210. doi: 10.4022/jafib.210.
- Robinson K, Frenneaux MP, Stockins B, Karatasakis G, Poloniecki JD, McKenna WJ. Atrial fibrillation in hypertrophic cardiomyopathy: a longitudinal study. J Am Coll Cardiol, 1990; 15: 1279-1285. 10.1016/s0735- doi: 1097(10)80014-2.
- Tendera M, Wycisk A, Schneeweiss A, Poloński L, Wodniecki J. Effect of sotalol on arrhythmias and exercise tolerance in patients with hypertrophic cardiomyopathy. Cardiology, 1993; 82: 335-342. doi: 10.1159/000175883.
- Moore JC, Trager L, Anzia LE, et al. Dofetilide for suppression of atrial fibrillation in hypertrophic cardiomyopathy: a case series and literature review. Pacing Clin Electrophysiol, 2018, 41: 396-401. doi: 10.1111/pace.13310.
- Jung H, Yang PS, Jang E, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest, 2019; 155: 354-363. doi: 10.1016/j.chest.2018.11.009.
- Di Donna P, Olivotto I, Delcrè SD, et al. Efficacy of catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: impact of age, atrial remodelling, and disease progression. Europace, 2010; 12: 347-355. doi: 10.1093/europace/euq013.
- Walters TE, Nisbet A, Morris GM, et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: implications for early ablative intervention. Heart Rhythm, 2016; 13: 331-339. doi: 10.1016/j.hrthm.2015.10.028.

