Transient Abnormal Myelopoiesis in Neonates
Ndeye Fatou Sow1,3, Abou Koundio2,*, Aminata Mbaye1,4, Abdoulaye Sene2, Cheikh Tidiane Ba2, Awa Ndiaye Ndir3, Diama Samb5, Mame Ndella Diouf3, Abibatou Sall5, Abou Ba1,3 and Awa Oumar Toure2
1Department of Paediatrics, Cheikh Anta Diop University, Senegal
2Hematology Laboratory, Cheikh Anta Diop University, Senegal
3Paediatrics Unit, Dalal Jamm Hospital, Senegal
4Paediatrics Unit, Albert Royer Children's Hospital, Senegal
5Hematology Laboratory, Dalal Jamm Hospital, Senegal
Received Date: 13/03/2025; Published Date: 28/04/2025
*Corresponding author: Abou Koundio, Hematology Laboratory, Cheikh Anta Diop University, Senegal
Abstract
Transient Abnormal Myelopoiesis (TAM) is a clonal pre-leukaemic neonatal syndrome. It is a pathological entity in patients with trisomy 21 that may go unrecognised, especially in its asymptomatic form. TAM is characterised by a clinico-biological presentation underpinned by specific genetic abnormalities that require appropriate management. We report the case of a newborn presenting with TAM at the paediatric oncology unit in Dakar, Senegal. The newborn was male, born to a 34-year-old mother in a well-monitored pregnancy, at term at 41 weeks’ gestation with a birth weight of 3640g. There was no consanguinity between the parents. Morphological examination of the newborn showed signs characteristic of the trisomy 21 phenotype. The haemogram at 3 days of age showed hyperleukocytosis with 147120 elements/mm3, normocytic normochromic anaemia with an hemoglobin level of 10g/dl, a platelet level of 345,000 elements/mm3 and a reticulocyte level of 96950 elements/mm3. The blood smear showed 84% blasts. The myelogram showed a type IV bone marrow characterised by the presence of medium-sized cells, often agranular, sometimes with fine granulations in cytoplasmic extensions. MPO was negative. The cytological picture was consistent with AML0. Immunophenotyping revealed a blastic population representing 22%, positive for CD33, CD34, CD117 and HLA-DR. The immunophenotypic profile was suggestive of AML with minimal maturation. The diagnosis of transient abnormal myelopoiesis was accepted. Management consisted of close monitoring, which returned to normal after one month. TAM requires diagnosis and appropriate management due to its potential complications, which can be fatal.
Keywords: Down syndrome; Myelopoiesis; Mother and child
Introduction
Myeloproliferative syndromes are a distinct disease entity when they occur in children with Down syndrome. These children have a unique predisposition to develop Acute Myeloid Leukaemia (AML) and a clonal neonatal pre-leukaemia syndrome known as Transient Abnormal Myelopoiesis (TAM). The latter may precede the onset of acute leukaemia or go into spontaneous remission. It is characterised by a natural history and a clinical and biological presentation underpinned by specific genetic abnormalities that require appropriate management. This disease is rare and poorly understood and often goes unrecognised, particularly in resource-limited countries where investigations may be compromised. We report the case of a neonate presenting with TAM at the paediatric oncology unit in Dakar, Senegal.
Case Presentation
Clinical features
This was a male newborn, born to a 34-year-old mother in a well-monitored pregnancy, at 41 weeks and 1 day of amenorrhoea, with a birth weight of 3,640g. There was no consanguinity between the parents. On clinical examination, the newborn had a head circumference of 34 cm and a height of 53 cm. There was a left parasternal holosystolic murmur radiating in the shape of a wheel radius and hepatomegaly with a regular surface. Morphological examination revealed features characteristic of the trisomy 21 phenotype, including a flat occiput, a short, broad neck, hypertelorism, upward and outward slanting palpebral fissures, epicanthus, and a flat nasal bridge. He had a protruding tongue, clinodactyly of the fifth finger, stocky hands with short, hyperlaxed fingers, a single transverse palmar fold, short, wide feet with a marked space between the hallux and the second toe, and axial and peripheral hypotonia. Karyotyping was not performed due to lack of funds. Doppler echocardiography revealed congenital heart disease with complete atrioventricular canal.
Laboratory findings
The blood count at 3 days of age showed hyperleukocytosis at 147120 elements/mm3, normocytic normochromic anaemia with haemoglobin (Hb) at 10 g/dl, platelets at 345,000 elements/mm3 and reticulocytes at 96950 elements/mm3 (Table I). The blood smear showed 84% blasts (Figure 1). Blood cultures and a white blood cell count were negative. The myelogram showed a rich type IV marrow without megakaryocytes, characterised by the presence of a majority of medium to large cells, often agranular, with a small proportion of cells containing fine granulations in cytoplasmic extensions (Figure 2). The other cell lines were mildly dysmorphic. Myeloperoxidase (MPO) staining was negative, leading to the conclusion that the cytology was consistent with AML0. Peripheral blood immunophenotyping showed the presence of a blast population representing 22% of the cells, with the following markers positive CD33, CD34, CD117 and HLA-DR. CD13, CD11b and CD16 were weakly positive. The monocyte markers CD14 and CD64 were negative. The immunophenotypic appearance was suggestive of AML with minimal maturation. A diagnosis of transient abnormal myelopoiesis was made.
Monitoring and follow-up
Management consisted of close monitoring of blood counts. The clinical course was characterised by regression of hepatomegaly. The white blood cell count returned to normal after one month (Table).
Table: Changes in haemogram parameters between D2 and D157 of life.
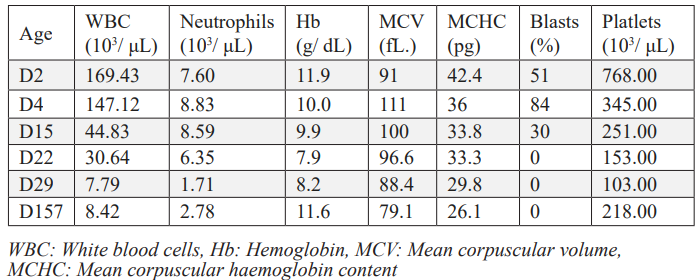
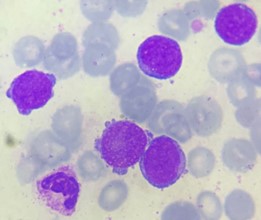
Figure 1: Small blasts in peripheral blood with a nucleus of fine multiple nucleoli chromatin and sparse basophilic and agranular cytoplasm, sometimes with processes.
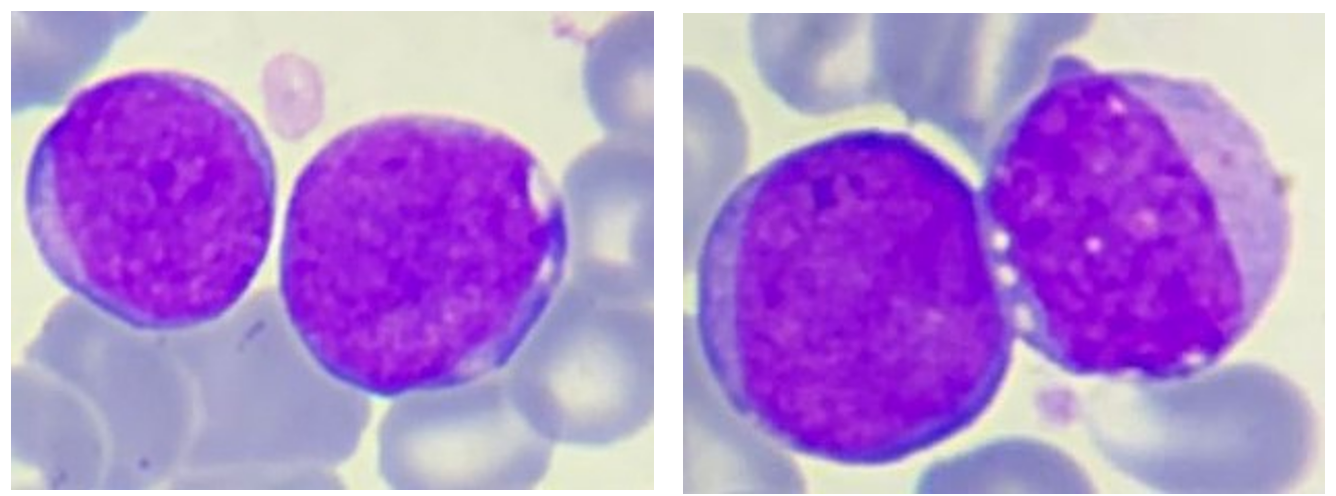
Figure 2: Small to medium-sized blasts: nucleus with fine chromatin and multiple nucleoli, a very basophilic and agranular cytoplasm with a very high nucleocytoplasmic ratio.
Discussion
Transient Abnormal Myelopoiesis (TAM) or Transient Myeloproliferative Disorder (TMD) is a pre-leukaemic syndrome that occurs in neonates or infants under 3 months of age and is characterised by the coexistence of trisomy 21 and a mutation in the GATA 1 gene [1].
Approximately 10% of newborns with trisomy 21 have TAM, with symptomatic forms allowing diagnosis, but also asymptomatic forms characterised by low levels of circulating blasts and identical genetic alterations [2]. The pathogenesis of this disease is still poorly understood, but is thought to be due to defects in fetal hepatic haematopoiesis caused by trisomy 21, in addition to acquired mutations in the GATA1 gene. GATA1 regulates the normal differentiation of megakaryocytes, erythroids, mast cells and eosinophils. The main mutations are located in exons 2 and 3.1 of the gene, resulting in the expression of a truncated GATA1s protein, which is found in all cases of TAM [3].
TAM can occur in a patient with a T21 phenotype, as the case in our observation, but also in a child with a normal phenotype with genetic mosaicism for T21 in the bone marrow [4].
The circumstances in which it is discovered are most often incidental or systematic during a polymalformative work-up.
Clinical manifestations vary from silent presentation with incidental diagnosis of haemogram and blood smear abnormalities to life-threatening complications. The diagnosis may be made during the clonal proliferation phase, at the time of complications, or during the spontaneous resolution phase. Therefore, it is important to systematically evaluate the haemogram and blood smear of every newborn with T21 [5].
Clinically, hepatomegaly and jaundice are the most common signs. Splenomegaly, skin lesions, pleurisy and pericarditis have also been described. Prolonged jaundice should raise suspicion of post-TAM hepatic fibrosis, which may be fatal [2].
Physical signs can be used to classify patients into different groups at risk of death. Three groups were identified. Patients without hepatomegaly or life-threatening complications were considered to be at low risk, with an overall survival rate of over 90%. Our patient with hepatomegaly alone was at intermediate risk. Patients with hepatomegaly and major complications were in the high-risk group [3]. Biologically, the main haematological abnormalities were hyperleukocytosis and high peripheral blasts, generally greater than 10%. Hyperleukocytosis is found in almost 50% of cases at the expense of neutrophils, myelocytes, monocytes and basophils [6-8].
Our patient presented with a severe hyperleukocytosis, which was detected with a significant peripheral blast count. There was also significant thrombocytosis. Variable platelet counts have been described in the literature. Abnormalities of fetal haematopoiesis in T21 include an increase in the number of megakaryocytic-erythroid precursors in the fetal liver and dysmegakaryopoiesis [9,10].
Anaemia is not common, but lower haemoglobin levels have been reported in newborns with TAM compared with newborns with T21 without TAM [7,8]. Blood cell abnormalities have been described in 20-25% of cases [4].
Megakaryoblastic blasts with basophilic cytoplasm, as in our patient, have been described in cases of TAM. Chopra et al. described agranular circulating megakaryoblasts. These different cytological characteristics were also found in our patient, although a wide variability in the morphology of the blasts was noted [6]. Similarly, immunophenotyping of the blasts shows variable profiles. They can show co-expression of stem cell markers (CD34 and CD117), myeloid markers (CD33/CD13), platelet glycoproteins (CD36, CD42, CD61) as well as CD56 and CD7 markers [11,12].
There is no consensus on the level of peripheral blasts required to diagnose TAM without identification of the GATA1 mutation [2]. Blast cells are found in the blood smear of the majority of patients with T21 and represent almost 15% of circulating leukocytes in neonates without the GATA1 mutation. Definitive diagnosis is based on the presence of more than 10% blasts associated with the presence of the GATA1 mutation [6].
This poses a challenge for the diagnosis of TAM, especially in resource-limited countries where genetic testing is difficult to access.
In our patient, the hepatomegaly resolved and haematological parameters returned to normal within the timescales described in the literature. Spontaneous resolution is generally observed. Consequently, therapeutic management consists of a “wait and see” approach with close monitoring, as was the case in our patient. Medical treatment is only initiated in the event of major complications: hydrops, organ failure, disseminated intravascular coagulation, cholestasis [13]. This is a short course of cytarabine-based chemotherapy aimed at reducing the leukaemic burden to allow resolution of symptoms. Depending on the study, the doses used vary between 0.4 and 1.5 mg/kg, administered intravenously or subcutaneously for 4 to 12 days. A reduction in complications and mortality has been reported with these protocols [8,14]. The majority of cases progress to spontaneous remission within 3 months of diagnosis. This is defined as complete resolution of symptoms, normalisation of haematological parameters and complete and permanent remission of the GATA1 mutation [2]. In 10-30% of cases, Transformation Disease (TD) progresses to Acute Myeloid Leukaemia (AML) within 5 years of diagnosis, with persistence of haematological abnormalities and acquisition of new oncogenic mutations. The median age of transformation was 16 months. Cytarabine treatment does not reduce the risk of progression to AML, for which the factors predicting transformation have not yet been elucidated [15,16].
Conclusion
TAM, especially in its asymptomatic form, is a pathological entity that may go unnoticed in patients with trisomy 21. It requires diagnosis and appropriate management because of the possible complications, which can be fatal. Systematic clinical and haematological surveillance of all newborns with trisomy 21 is essential to detect TAM and to diagnose any resulting AML at an early stage.
References
- Lange B. The management of neoplastic disorders of haematopoiesis in children with Down's syndrome. Br J Haematol, 2000; 110 (3): 512-524.
- Bhatnagar N, Nizery L, Tunstall O, Vyas P, Roberts I. Transient Abnormal Myelopoiesis and AML in Down Syndrome: An Update. Curr Hematol Malig Rep, 2016; 11(5): 333–341.
- Gamis AS, Alonzo TA, Gerbing RB, et al. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children's Oncology Group Study A2971. Blood, 2011; 118(26): 6752-6759.
- Massey GV, Zipursky A, Chang MN et al. A prospective study of the natural history of transient leukemia (TL) in neonates with Down syndrome (DS): Children's Oncology Group (COG) study POG-9481. Blood, 2006; 107(12): 4606-4613.
- Chalia M, Seager E, Rao A, Hannam. Transient abnormal myelopoiesis requiring advanced neonatal intensive care treatment. Acta Paediatr, 2024; 113(5): 980-988.
- Roberts I, Alford K, Hall G, et al. GATA1-mutant clones are frequent and often unsuspected in babies with Down syndrome: identification of a population at risk of leukemia. Blood, 2013; 122: 3908–3917.
- Klusmann J-H, Creutzig U, Zimmermann M, Dworzak M, et al. Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood, 2008; 111: 2991–2998.
- Gamis AS, Alonzo T, Gerbing R, et al. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children’s Oncology Group Study A2971. Blood, 2011; 118: 6752–6759.
- Tunstall-Pedoe O, Roy A, Karadimitris A, et al. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood, 2008; 112: 4507–4511.
- Roy A, Cowan G, Mead AJ, et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci U S A, 2012; 109: 17579–17584.
- Karandikar NJ, Aquino DB, McKenna R, et al. Transient myeloproliferative disorder and acute myeloid leukemia in Down syndrome. An immunophenotypic analysis. Am J Clin Pathol, 2001; 116: 204–210.
- Boztug H, Schumich A, Potschger U, et al. Blast cell deficiency of CD11a as a marker of acute megakaryoblastic leukaemia and transient myeloproliferative disease in children with and without Down syndrome. Cytometry B Clin Cytom, 2013; 84: 370–378.
- Park M, Sotomatsu M, Ohki K, et al. Liver disease is frequently observed in Down syndrome patients with transient abnormal myelopoiesis. Int J Hematol, 2014; 99: 154–161.

