A 57-Year-Old Female with Differentiated High-Grade Thyroid Carcinoma with Occult Metastasis to the Cerebellum Revealed After rhTSH Administration
Aleksandra Ledwon1,*, Ewa Paliczka-Cieślik1, Aleksandra Kropińska1, Aleksandra Kukulska1,2 and Daria Handkiewicz-Junak1
1Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Gliwice Branch, Wybrzeże Armii Krajowej 15, 44-102 Gliwice, Poland
2Radiotherapy Department, Maria Sklodowska-Curie National Research IOnstitute of Oncology, Gliwice Branch, Wybrzeże Armii Krajowej 15, 44-102 Gliwice, Poland
Received Date: 20/01/2025; Published Date: 18/02/2025
*Corresponding author: Aleksandra Ledwon, Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Gliwice Branch, Wybrzeże Armii Krajowej 15, 44-102 Gliwice, Poland
Abstract
To treat thyroid cancer with radioiodine high TSH level is necessary. It can be achieved by administration of recombinant human TSH (rhTSH). The most common side effects of rhTSH are headaches, nausea and vomiting. However, there are reports of patients with local recurrence or distant metastases who had severe complications after rhTSH administrations.
We present a case of 57-year-old woman with differentiated high-grade thyroid carcinoma treated with RAI under rhTSH stimulation. The patients had no known distant metastases and presented no neurological symptoms. 24 hours after the first dose of rhTSH the patient reported nausea and vomiting which were increasing despite treatment. The CT scan revealed a pathological lesion in the cerebellum accompanied by oedema and mass effect with beginning of foraminal herniation. Urgent surgical intervention was necessary. We present this case to highlight that, although nausea and vomiting are recognized side effects of rhTSH, these symptoms can occasionally indicate the sudden onset of manifestations caused by occult central nervous system metastases.
Keywords: DHGTC; rhTSH; Occult metastases to cerebellum
Introduction
Differentiated high-grade thyroid carcinoma (DHGTC) was officially classified as a distinct diagnostic category, separate from poorly differentiated thyroid carcinoma (PDTC), in the 2022 WHO classification of thyroid tumors. [1,2]. Both PDTC and DHGTC demonstrate an intermediate clinical phenotype between well differentiated thyroid carcinoma and anaplastic thyroid carcinoma. [2,3]. During clinical examination DHGTC usually exhibits advanced local disease, with large invasive tumors, extrathyroidal extension and distant metastases at the time of diagnosis ranging from 6% to 40% [2-5]. DHGTC most commonly metastasizes to the lungs, bones and mediastinum [3,6,7]. Surgical resection is the cornerstone of treatment in patients with DHGTC [8]. The postoperative use of radioiodine (RAI) in patients with DHGTC is recommended due to the potential benefit and minimal side effects [4,9]. Administration of RAI requires TSH stimulation, which may be achieved by two possible methods: (1) L-thyroxin withdrawal (THW) to provoke endogenous TSH elevation or (2) exogenous stimulation with recombinant human TSH (rhTSH). Several studies emphasized that rhTSH stimulation is well-tolerated and associated with better quality of life [10]. The use of rhTSH has been advocated in patients with brain or spinal metastases to avoid chronic endogenous TSH stimulation of neoplastic tissue, which could predispose the tumor expansion [11]. However, there have been case reports of neurological complications associated with the use of rhTSH in patients with metastases to the brain, spine or vertebrae [12].
Case Report
We present a case of 57-year-old woman with differentiated high-grade thyroid carcinoma pT3bN0M1 LVI1 PNI0 R1 AJCC ed.8. The patient underwent non-radical surgery in May 2023. She was than was referred to Institute of Oncology in Gliwice, however, due to rapid and extensive local progression of the disease stated in CT scan in 11.2023 (recurrence in the left thyroid bed descending to upper mediastinum, infiltration of the left common carotid artery, esophagus and trachea) she was disqualified from reoperation. Subsequently EBRT was administered to stabilize disease – from December 2023 to February 2004 the patient received 50Gy to the left cervical lymph nodes and mediastinum, 60 Gy to the thyroid bed and boost of 72 Gy to the recurrence.
Molecular analysis revealed no mutations in genes connected with novel targeted therapies.
Then the patient was admitted to The Department of Nuclear Medicine and Endocrine Oncology on 24th April 2024 with the intention to treat with 131I. During the clinical interview, the patient did not report any neurological symptoms. However, after administration of the first dose of rhTSH she manifested nausea and uncontrollable vomiting, not responding to standard treatment with metoclopramide and ondansetron. We decided to perform non-contrast CT scan, which revealed a pathological lesion in the cerebellum accompanied by oedema and mass effect with beginning of foraminal herniation (Figure 1A). The patient was immediately referred to the neurosurgical ward, where urgent ventriculostomy was performed, general condition of the patient improved, and the symptoms subsided. Local disease in the neck was stable, control CT with contrast showed regression of hydrocephalus and brain oedema (Figure 1B). Under the cover of anti-edematous medication, she was qualified for planned neurosurgery and on June 20th, 2024, the cerebellum tumor was removed (histopathological examination confirmed metastasis from thyroid cancer). Subsequent stereotactic radiotherapy of 25Gy in 5 fractions to the tumor bed was performed 2 months later. Up to now, the patient received two radioiodine treatments with the cumulative dose of 200 mCi / 7400 MBq. The posttherapy whole-body scan performed 72 hours after administration of 100 mCi / 3700 MBq 131I showed radioiodine accumulation in thyroid bed and residual infiltration in the left mediastinum (Figure 2A, 2B). Until now the patient stays in a good general condition without any neurological symptoms and the latest MRI scan showed no pathological findings in CNS, thyroglobulin serum level (under TSH suppression) decreased from 114 ng/ml in April 2024 to 0,5 ng/ml in January 2025. Neck and chest CT check-up as well as the third radioiodine treatment are planned.
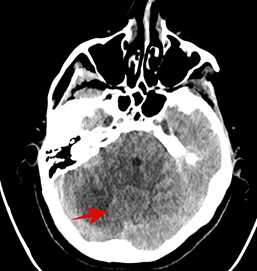
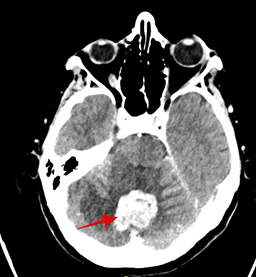
Figure 1: (A) Non-contrast CT (25/04/2024) scan (B) contrast CT (8/05/2024), shows a pathological lesion in the cerebellum.
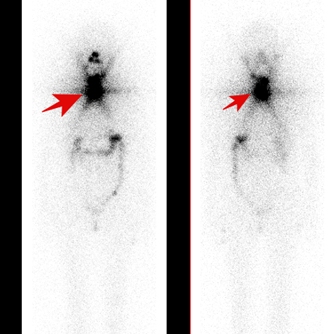
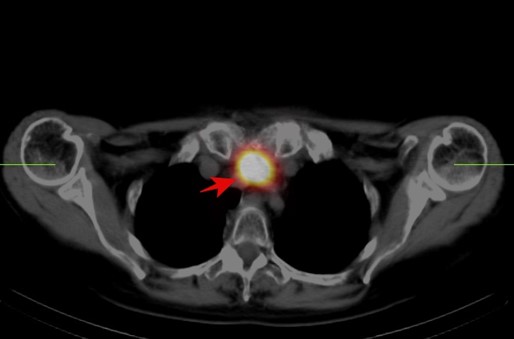
Figure 2: (A) Posttherapeutic 131I planar whole-body scan showed radioiodine uptake in thyroid bed and residual infiltration in the left mediastinum (B) Image fusion of CT and iodine SPECT scan of the thoracic region showed radioiodine uptake in residual infiltration in the left mediastinum.
Discussion
It is generally assumed that administration of rhTSH may mitigate the risk of tumor swelling associated with endogenous TSH stimulation by significantly shortening the duration of TSH elevation. It is also well-tolerated. The most common side effects associated with rhTSH administration are headaches, nausea and vomiting. These symptoms are mild and transient [13].
However, the peak values of TSH after rhTSH are significantly higher than after thyroid hormone withdrawal, and there is a potential increased risk of tumor swelling after rhTSH. There are literature data reporting severe complications following rhTSH administration. Robbins et al described a patient with multiple bone and brain metastases who developed confusion, ataxia, dysphagia and headache after rhTSH administration [11]. However, the patient was previously treated after thyroid hormone withdrawal which was associated with sudden onset of hemiparesis. The complications resulted from increased oedema surrounding the brain metastasis. Braga et al described two patients with local recurrent tumor, who developed tumor growth 12-48 hours after the second rhTSH injection, reflected by acute respiratory distress or a palpable, tender mass [14]. Goffman et al reported a case of patient who developed near-lethal respiratory failure after rhTSH [15].
The patient we present had no known distant metastases at the time of preparation for radioiodine treatment, nor did she present any symptoms suggesting the presence of brain metastases. She developed nausea and then uncontrollable vomiting 24 hours after the first dose of rhTSH. Nausea and vomiting are the most common side effects of rhTSH, but they subside after antiemetic drugs. In our case, the patient's symptoms progressed despite ongoing treatment. That was the reason we decided to perform CT scan (without contrast due to planned radioiodine therapy), which revealed a pathological lesion in the cerebellum accompanied by oedema and mass effect with beginning of foraminal herniation. The patient's symptoms were undoubtedly caused by swelling and tumor enlargement caused by the administration of rhTSH. It cannot be ruled out that the neurological symptoms would also occur following thyroid hormone withdrawal, but perhaps with more gradual onset.
Conclusion
We present this case to emphasize that in patients at high risk of distant metastases, nausea and vomiting following rhTSH administration, particularly if persistent or worsening despite treatment, should raise concern for potential brain metastases as a contributing factor. In such cases, imaging studies should be promptly performed to assess the need for urgent surgical intervention. Additionally, glucocorticoid coverage should be considered during rhTSH administration in patients with known or suspected tumors in confined anatomical regions to mitigate potential complications.
Author contributions
Aleksandra Ledwon: Conceptualisation, literature review, writing original draft, review and editing
Ewa Paliczka-Cieślik: Conceptualisation, literature review, review,
Aleksandra Kropińska: review and editing
Aleksandra Kukulska: review and editing
Daria Handkiewicz-Junak: review, editing, supervision
The authors declare no conflict of interest.
The work should be credited to: Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie National Research Institute of Oncology, Gliwice Branch, Poland
References
- Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasm. Endocr Pathol, 2022; 33(1): 27-63. doi: 10.1007/s12022-022-09707-3.
- Christofer Juhlin C, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel cocepts in nomenclature and grading. Endocr Relat Cancer, 2022; 30(2): e220293. doi: 10.1530/ERC-22-0293.
- Wong KS, Dong F, Telatar M, Lorch JH, Alexander EK, Marqusee E, et al. Papillary Thyroid Carcinoma with High-Grade Features Versus Poorly Differentiated Thyroid Carcinoma: An Analysis of Clinicopathologic and Molecular Features and Outcome. Thyroid, 2021; 31(6): 933-940. doi: 10.1089/thy.2020.0668.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid, 2016; 26(1): 1-133. doi: 10.1089/thy.2015.0020.
- Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid, 2019; 29(3): 311-321. doi: 10.1089/thy.2018.0509.
- Sanders EM Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg, 2007; 31(5): 934-945. doi: 10.1007/s00268-007-9033-3.
- Tondi Resta I, Gubbiotti MA, Montone KT, Livolsi VA, Baloch ZW. Differentiated high grade thyroid carcinomas: Diagnostic consideration and clinical features. Hum Pathol, 2024: 144: 53-60. doi: 10.1016/ j.humpath.2024.01.002.
- Alam IS, Patel KN. Management of Poorly Differentiated Thyroid Cancer and Differentiated High-Grade Thyroid Carcinoma. Surg Clin North Am, 2024; 104(4): 751-765. doi: 10.1016/j.suc.2024.02.005.
- Tong J, Ruan M, Jin Y, Fu H, Cheng L, Luo Q, et al. Poorly differentiated thyroid carcinoma: a clinician's perspective. Eur Thyroid J, 2022; 11(2): e220021. doi: 10.1530/ETJ-22-0021.
- Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Potential use of recombinant human thyrotropin in the treatment of distant metastases in patients with differentiated thyroid cancer. Endocr Pract, 2013; 19(1): 139-48. doi: 10.4158/EP12244.RA.
- Robbins RJ, Voelker E, Wang W, Macapinlac HA, Larson SM. Compassionate use of recombinant human thyrotropin to facilitate radioiodine therapy: case report and review of literature. Endocr Pract, 2000; 6(6): 460-464. doi: 10.4158/EP.6.6.460.
- Vargas GE, UY H, Bazan C, Guise TA, Bruder JM. Hemiplegia after thyrotropin alfa in a hypothyroid patient with thyroid carcinoma metastatic to the brain. J Clin Endocrinol Metab, 1999; 84(11): 3867-371. doi: 10.1210/jcem.84.11.6161.
- Meier CA, Braverman LE, Ebner SA, Veronikis I, Daniels GH, Ross DS, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study). J Clin Endocrinol Metab, 1994; 78(1): 188-196. doi: 10.1210/jcem.78.1.8288703.
- Braga M, Ringel MD, Cooper DS. Sudden enlargement of local recurrent thyroid tumor after recombinant human TSH administration. J Clin Endocrinol Metab, 2001; 86(11): 5148-5151. doi: 10.1210/jcem.86.11.8055.
- Goffman T, Ioffe V, Tuttle M, Bowers JT, Mason ME. Near-lethal respiratory failure after recombinant human thyroid-stimulating hormone uses in a patient with metastatic thyroid carcinoma. Thyroid, 2003; 13(8): 827-830. doi: 10.1089/105072503768499734.

