The Benefits of PCSK9 Inhibition in a Patient with Presumed Familial Hypertriglyceridemia: Significant Reduction of Cardiovascular Risk
Oana-Andreea Parlițeanu1, Octavian-Sabin Alexe2, Cristiana Voineag2,*, Daniel Radu3, Loredana Mitral5, Roxana Maria Nemes1 and Beatrice Mahler1,4
1National Institute of Penumoftiziology “Marius Nasta” Bucharest, Romania
2“Dunarea de Jos” University of Medicine, Galati, Romania
3Ilfov County Emergengy Clinical Hospital, Romania
4“Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
5“Elias” Univeristy Emergency Hospital, Bucharest, Romania
Received Date: 17/10/2024; Published Date: 07/11/2024
*Corresponding author: Cristiana Voineag, ”Dunarea de Jos” University of Medicine, Galati, Romania
Introduction
Familial Hypertriglyceridemia (FHTG) is a genetic disorder characterized by markedly elevated triglyceride levels in the blood. This condition can lead to severe complications, including pancreatitis and cardiovascular diseases [1,2]. Traditional lipid-lowering therapies, such as statins, may not be effective for all patients with FHTG. Recent advancements in pharmacotherapy have introduced PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors as a novel treatment option. This article explores the benefits of PCSK9 inhibition for patients suffering from familial hypertriglyceridemia, focusing on its mechanism of action, clinical efficacy, safety profile, and overall impact on patient outcomes.
We will present the case of a 46-year-old man, a smoker, 20 pack per year, that does not consume alcohol or drugs, which presented in our office with extreme values for his lipid panel. The patient had no relevant medical history or history of familial dyslipidemia.
Patient was obese with a BMI (body mass index) of 30.71kg/m, with abdominal obesity, waist circumference 102cm.
In 2018, when he first came into our office, he had the following lipid profile: total cholesterol 367mg/dl (9.5mmol/L), HDL Cholesterol 16mg/dl (0.4mmol/L), LDL Cholesterol could not be calculated at that time, Triglycerides 2716mg/dl (30.7mmol/L).
Figure 1: Patient`s blood after 10 minutes after it was collected.
Understanding Familial Hypertriglyceridemia
Familial hypertriglyceridemia is primarily caused by genetic mutations affecting triglyceride metabolism. These mutations can occur in genes involved in lipid transport and metabolism, such as the Lipoprotein Lipase (LPL) gene [3,4]. The condition is characterized by fasting triglyceride levels exceeding 200 mg/dL, often leading to severe complications if left untreated.
Patients with FHTG frequently experience recurrent pancreatitis, which can result in significant morbidity and mortality [5]. Moreover, the elevated triglyceride levels contribute to an increased risk of atherosclerosis and cardiovascular diseases [6]. Given the complexities associated with managing FHTG, there is a critical need for effective therapeutic options.
After initially evaluating our patient, we thought that his diagnosis is FHTG, since the triglycerides were over 2000mg/dl, but we could not find any other clues that this was the correct diagnosis; a limitation in our cause was the lack of genetic testing in our country at that time. We evaluated the Risck of cardiovascular event (coronary or stroke death or nor fatal myocardial infarction) in the next 10 years, and the risk was very high: 45.6%.
Initially we started the patient on very high dose of statin (rosuvastatin 40mg qd), fenofibrate 160mg qd, omega 3 acid 1000mg qd and acetylsalicylic acid 75mg qd; also, a low fat and low in simple carbs diet was explained to the patient, along with the recomandation of not using alcohol. This was the initial treatment, which was followd intermittently by the patient from 2018 to 2023.
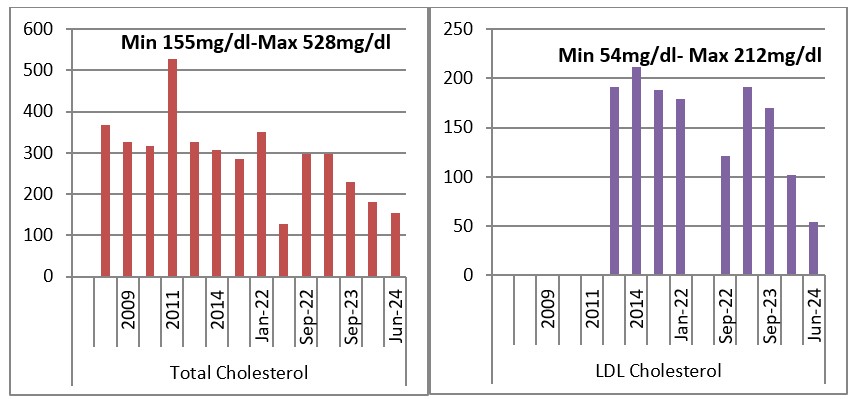
Graph 1 and 2: Evolution of Total Cholesterol and LDL Cholesterol 2018-2024.
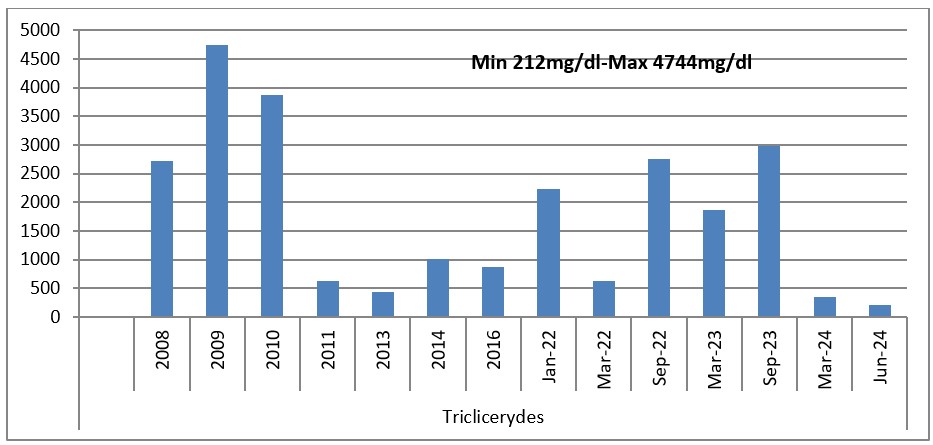
Grapg 3: Evolution of Triglycerides 2018-2024.
In this interval 2018-2023, the patient went “up & down” with his lipid values according to the diet and medication that he adhered or not to. In 2009 and 2010, where the triglycerides and cholesterol values were the highest; tryglicerides of 4744mg/dl (53.6mmol/L) and Total cholesterol was 528mg/dl (13.7mmol/l), a switch between rosuvastatin 40mg qd was tried with atorvastatin 80mg qd. Unfortunately, the patient did not tolerate atorvastatin 80mg, he complained of myalgia and had an elevated CK (creatine kinase) and he was switched back to rosuvastatin 40mg qd.
In 2022 when the patient presented again with high values of lipid panel, Ezetimib 10mg was added to his medication along with rosuvastatin 40mg qd, fenofibrate 145mg qd, omega 3 acid 1000mg qd and acetylsalicylic acid 75mg qd. Patient complained of stomach discomfort, dizziness and myalgia, therefore the treatment with ezetimibe was discontinued. At this point an increase in the dose of omega 3 1000mg was preformed, from one tablet a day to four tablets a day, adding up to 4000mg/day. This treatment unfortunately is not reimbursed and the patient could only afford it for a limited period of time, usually taking two maximum three per day.
The evolution of LDL Cholesterol, when it could be calculated was similar to that of the Total Cholesterol and Triglycerides, and since 2022 we were able to measure LDL Cholesterol directly, which was a great tool in evaluating the cardiovascular risk.
In 2023, the patient was initiated with Alirocumab 75mg one injection every two weeks, a new PCSK-9 inhibitor that was available from that time also in Romania.
Mechanism of Action of PCSK9 Inhibitors
PCSK9 inhibitors are a class of monoclonal antibodies that target the PCSK9 protein, which plays a key role in cholesterol metabolism. PCSK9 binds to Low-Density Lipoprotein Receptors (LDLR) on hepatocytes, leading to their degradation. This process reduces the number of LDL receptors available to clear circulating LDL cholesterol from the bloodstream [7,8].
By inhibiting PCSK9, these medications increase the availability of LDL receptors, enhancing the liver's ability to remove LDL cholesterol from the circulation. This mechanism not only lowers LDL cholesterol levels but may also influence triglyceride levels, making PCSK9 inhibitors a promising option for patients with hypertriglyceridemia [9,10].
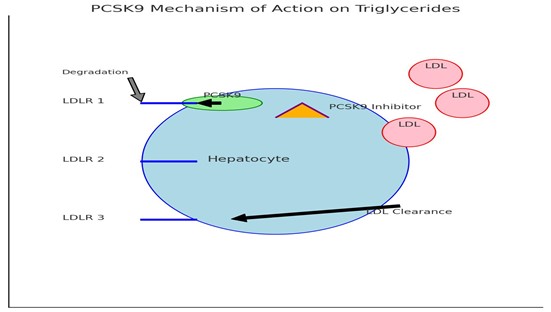
Graph 4: PCSK-9 Mechanism of action on Triglycerides.
Clinical Efficacy of PCSK9 Inhibitors in Familial Hypertriglyceridemia
Recent clinical trials have demonstrated the efficacy of PCSK9 inhibitors in patients with elevated triglyceride levels, including those with familial hypertriglyceridemia. Studies have shown that these agents can lead to significant reductions in triglyceride levels, often in conjunction with LDL cholesterol reduction.
One notable trial investigated the effects of a PCSK9 inhibitor on patients with FHTG and observed a substantial decrease in triglyceride levels, with a mean reduction of approximately 25-40% [11]. Additionally, improvements in other lipid parameters, such as non-HDL cholesterol and apolipoprotein B levels, were reported [12].
These findings highlight the dual benefit of PCSK9 inhibition in managing both LDL cholesterol and triglyceride levels, offering a comprehensive approach to lipid management in FHTG patients.
For our patient the treatment the introduction of Alirocumab 75mg one injection every two weeks translated into a reduction of Total Cholesterol, LDL Cholesterol and Triglycerides, but unfortunately the reduction was not as we expected and we decide to titrate the dose and increase at 150mg one injection every two weeks in 2024.
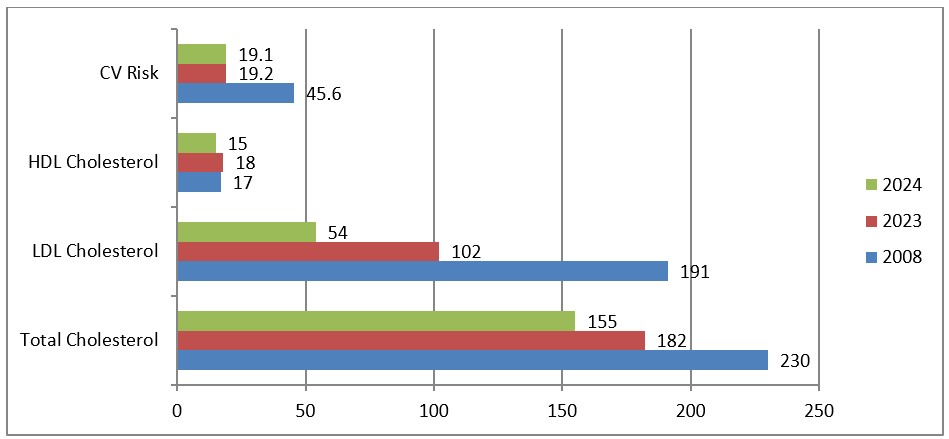
Graph 5: Evolution of lipid panel and cardiovascular risk 2018-2024.
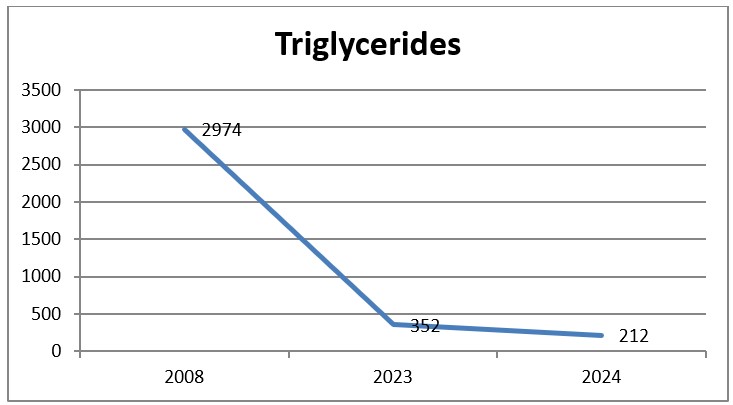
Graph 6: Evolution of triglycerides 2018-2024.
After the rising of the dose in Alirocumab from 75 to 150mg once every two weeks, the patient presented with the lowest values in Total Cholesterol 155mg/dl (4.0mmol/L), in LSL Cholesterol 54mg/dl (1.4mmol/L), in triglycerides 212mg.dl (2.4mmol/L), also the patient presented a reduction of the cardiovascular risk from 45.6 to 19.1.
Safety Profile of PCSK9 Inhibitors
The safety profile of PCSK9 inhibitors is generally favorable, with most patients experiencing minimal side effects. Common adverse reactions include injection site reactions, fatigue, and myalgia; however, these are typically mild and transient [13].
Importantly, large-scale studies have not demonstrated a significant increase in cardiovascular events associated with PCSK9 inhibitors. The lack of adverse outcomes combined with their effectiveness in lowering lipid levels makes them a viable treatment option for patients with FHTG, particularly those who are intolerant to traditional therapies [14,15].
Impact on Patient Outcomes
The introduction of PCSK9 inhibitors into the treatment landscape for familial hypertriglyceridemia has the potential to significantly improve patient outcomes. By effectively managing lipid levels, these agents can reduce the risk of acute pancreatitis and cardiovascular events, ultimately enhancing quality of life.
Furthermore, the ability to achieve target triglyceride levels may reduce the need for hospitalization due to pancreatitis and decrease healthcare costs associated with managing complications of FHTG [6, 16]. This holistic approach underscores the importance of incorporating PCSK9 inhibitors into treatment regimens for patients with familial hypertriglyceridemia.
Conclusion
Familial hypertriglyceridemia poses significant challenges in clinical management due to its genetic basis and associated complications. The emergence of PCSK9 inhibitors has revolutionized the treatment paradigm, offering a powerful tool for effectively managing lipid levels in these patients. Through their mechanism of action, clinical efficacy, and favorable safety profile, PCSK9 inhibitors present a promising option for individuals with FHTG. As research continues to unfold, the integration of these agents into standard care may lead to improved outcomes and quality of life for patients grappling with this challenging condition.
The use of PCSK9 inhibitors in our patient resulted in a 26.5% reduction in the cardiovascular risk, the reduction and normalization on Total Cholesterol and LDL Cholesterol and also an increase in HDL Cholesterol and a reduction almost to the normal levels of triglycerides from 2974mg/dl (33.6mmol/l) to 212mg/dl (2.4mmol/L).
References
- Bottorff C, et al. Familial Hypertriglyceridemia: A Comprehensive Review. Journal of Lipid Research, 2020; 61(2): 210-220.
- Miller M, et al. Familial Hypertriglyceridemia: Pathophysiology and Treatment. Cardiology Clinics, 2019; 37(1): 1-16.
- Huang Y, et al. Genetic Basis of Familial Hypertriglyceridemia. Nature Reviews Genetics, 2021; 22(8): 551-570.
- Le May C, et al. Familial Hypertriglyceridemia: Clinical Perspectives and Management. Journal of Clinical Lipidology, 2020; 14(5): 535-542.
- Sinha A, et al. Risk of Cardiovascular Events in Patients with Familial Hypertriglyceridemia: A Review of Current Evidence. Clinical Lipidology, 2019; 14(4): 309-319.
- Nordestgaard BG, et al. Triglyceride-Rich Lipoproteins and Risk of Cardiovascular Disease. Journal of Internal Medicine, 2016; 280(5): 453-469.
- Ghosh S, et al. Mechanisms of PCSK9 Inhibition and its Role in LDL Reduction. Current Atherosclerosis Reports, 2020; 22(9): 61.
- Yu J, et al. PCSK9 and Its Role in Lipid Metabolism: A Review. Journal of Lipid Research, 2018; 59(10): 1775-1788.
- Ding Y, et al. PCSK9 Inhibitors: Mechanisms of Action and Clinical Benefits. Cardiovascular Drugs and Therapy, 2022; 36(3): 265-274.
- Tsimikas S, et al. The Role of PCSK9 in Triglyceride Metabolism: Implications for Cardiovascular Disease Management. Atherosclerosis, 2019; 283: 75-82.
- Kostapanos M, et al. The Impact of PCSK9 Inhibition on Triglyceride Levels: A Meta-Analysis of Clinical Trials. Atherosclerosis, 2020; 296: 32-38.
- Fischer S, et al. Effects of PCSK9 Inhibitors on Lipid Levels in Patients with Familial Hypertriglyceridemia. European Heart Journal, 2021; 42(14): 1405-1413.
- Robinson JG, et al. Efficacy and Safety of Evolocumab in Patients with Hypercholesterolemia: A Randomized Clinical Trial. Journal of the American Medical Association, 2016; 316(19): 1994-2003.
- Cohen J, et al. Long-Term Safety of PCSK9 Inhibitors: A Systematic Review and Meta-Analysis. Atherosclerosis, 2017; 259: 1-10.
- Sabatine MS, et al. Efficacy of Alirocumab in Reducing Lipids and Cardiovascular Events. New England Journal of Medicine, 2017; 376(15): 1430-1440.
- Sweeney MN, et al. PCSK9 Inhibitors and Their Role in Clinical Practice. Current Opinion in Lipidology, 2020; 31(6): 487-493.

