Testicular Masses in Long Standing Congenital Adrenal Hyperplasia Boy: A Diagnosis Dilemma
Randa I1 * and Nermine HA1
1Department of Pediatrics, Endocrinology clinic, Children’s Hospital, Ain shams University, Cairo, Egypt
Received Date: 30/06/2020; Published Date: 22/07/2020
*Corresponding author: Randa I. Khalaf, Department of Pediatrics, Endocrinology clinic, Children’s Hospital, Ain shams University, 28 Mohamed Tawfik Diab, Cairo, Egypt. Tel: 201001102030; Fax: 226709709; E-mail: randakhalaf1@hotmail.com
Abstract
Congenital adrenal hyperplasia (CAH) refers to group of inherited diseases resulting from impaired adrenal steroidogenesis, and the most common cause is 21-hydroxylase enzyme deficiency. Genital examination must be done to all patients with CAH at every follow up visit. Testicular masses as testicular adrenal rest tumors (TART) and leydig cell hyperplasia or tumors may appear occasionally in both adequately treated and untreated cases. We report a case of bilateral leydig cell tumor in a 6-year-old male patient with lately diagnosed simple virilizing form of CAH. He presented with gradually increasing asymmetric bilateral testicular masses. Testosterone levels were high despite receiving appropriate dose of glucocorticoids and controlling the puberty advancement. Testicular biopsy showed bilateral leydig cell tumor. Testis sparing surgery was done after which testosterone normalized.
Keywords: CAH; Testicular Masses; Leydig Cell Tumors
Abbreviations: CAH: Congenital Adrenal Hyperplasia; TART: Testicular Adrenal Rest Tumors; ACTH: Adrenocorticotropic Hormone; LCTs: Leydig Cell Tumors; SD: Standard Deviation; Na : Sodium; K : Potassium; DHEA : Dehydroepiandrosterone; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; LHRH: Lutenizing Hormone Releasing Hormone; HCG: Human Chorionic Gonadotropin; CD: Cluster of Differentiation; MRI: Magnestic Resonance Imaging
Introduction
Testicular masses in cases of congenital adrenal hyperplasia (CAH) were originally described in 1940s. They are known as testicular tumors of the adrenogenital syndrome or testicular adrenal rest tumours (TARTs) arising in almost 94% of CAH adults and may appear in children [1]. TARTs originate due to prolonged adrenocorticotropic hormone (ACTH) hypersecretion from old adrenal cells present in the testes during embryogenesis. They are often small and may be overlooked in genital examination but become evident when they increase in size becoming palpable or causing dragging pain in the scrotum. The etiology of leydig cell tumors (LCTs) remains unknown. They are not associated with cryptorchidism. The disruption of the hypothalamic-pituitary testicular axis by excess luteinizing hormone may induce Leydig cells oncogenesis [2]. Due their completely different therapeutic approaches, endocrinologists face a clinical challenge to differentiate between them with the help of imaging and histopathological studies.
Case Presentation
A 6-year-old male patient presented with progressively enlarging genitalia over a period of 1 year. On examination, his height was 104.5 cm (+3SD) and bone age was 8.5 years, Tanner staging: P3, penile length was 8.5cm and testes were 4ml bilaterally.
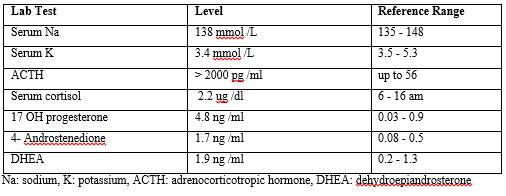
Laboratory investigations shown in (Table 1) (Figure 1)
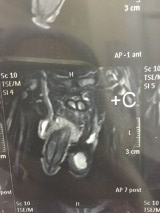
Figure 1: Scrotal MRI: Lobulated exophytic soft tissue masses replacing most of testes parenchyma with thin mantle of testicular tissue extending to epididymis & spermatic cord with hydrocele and varicocele.
Table 1: Laboratory results
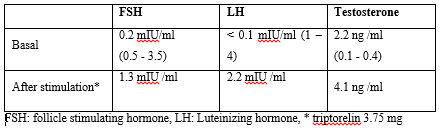
The results of hormonal profile as shown in (Table 2) were consistent with the diagnosis of isosexual peripheral precocious puberty complicating untreated lately diagnosed simple virilizing form of CAH. The patient was started on regular daily oral hydrocortisone (12.5mg/m2). Height and puberty were advancing very rapidly despite of proper compliance to hydrocortisone so Lutenizing hormone releasing hormone (LHRH) analogue was started monthly after which testosterone level decreased to 0.47pg/ml. Six months later both testes increased to 12 ml and testosterone level increased to 5.8 ng/ml. At this point the dose of hydrocortisone was modified to 15mg/m2 and testosterone levels went down to 0.34 pg/ml. 6 months later despite giving the stronger suppressive dose of hydrocortisone, the patient presented with further enlarged testes size (20 ml) with multiple palpable scrotal swellings more on the right side and serum testosterone was 5.8 ng/ml. (Table 2) (Figure 2)
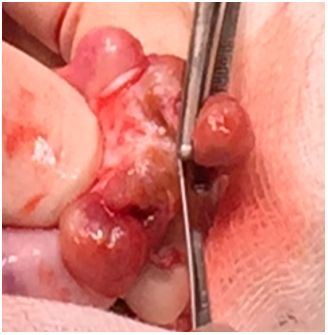
Figure 2: Gross examination of the orchiectomy specimen: multiple lobulated testicular lesions (brown in cut section).
Table 2: Hormonal profile
Scrotal ultrasound revealed bilateral soft lesions above the testes with homogenous echogenicity and no focal lesions confirming the presence of TARTs. Alpha-feto protein and human chorionic gonadotropin (HCG) levels were normal. Scrotal magnetic resonance imaging (MRI) showed the presence of lobulated exophytic soft tissue masses replacing most of testes parenchyma with thin mantle of testicular tissue left around. The masses extended to epididymis & spermatic cord with hydrocele and varicocele concluding the diagnosis of leydig cell tumors and excluding TARTs. After the contradicting results of the two imaging techniques, fine needle aspiration and cytology was done. The specimen showed the proliferation of leydig cells with eosinophilic cytoplasm and prominent nuclei arranged in nodules with prominent fibrous tissue septae. There were no reinke crystals seen. The cells showed negative staining to synaptophysin and positively stained with inhibin. This was consistent with the diagnosis of bilateral Leydig cell tumor and not TARTs. The patient was referred for a testis sparing surgery. Both testes grossly showed varicocele, hydrocele and the appearance of multiple well-defined rounded brown masses. Under microscopy they showed the same previously mentioned criteria confirming again the presence of bilateral leydig cell tumor. The testosterone dropped to 0.22 ng / ml after the successful surgical resection of the masses. Last follow up of the patient at the age of 8 years shows that his height is at 143.8 cm (+ 3.2 SD) with height velocity of 4.8 cm per year, testes were 4ml and 17 OH progesterone was 0.95 ng/ml on 15 mg/m2 hydrocortisone.
Discussion
Late diagnosis of simple virilizing form of CAH may predispose the patient to precocious puberty and the development of testicular adrenal rest tumors due to the prolonged exposure to adrenal androgens [3]. It has been shown that most of those cases present with the CAH before the appearance of the testicular masses. Differential diagnosis of testicular masses arising in CAH cases may include TARTs and leydig cell hyperplasia or tumors [4]. (Figure 3)
Despite of the benign nature of TARTs, they must be differentiated from the leydig cell hyperplasia and tumors to avoid unnecessary surgical procedures. They can be often misdiagnosed as the more dangerous leydig cell tumor especially when the lesions are resistant and do not subside even after proper control of the CAH [5].
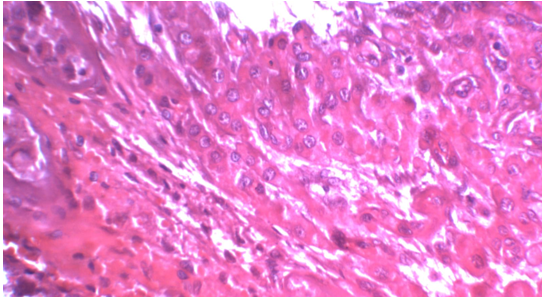
Figure 3: Frozen sections: The lesion was composed of sheets and nests separated by dense fibrous tissue. The individual cells were large round and polygonal cells with defined cell borders, abundant eosinophilic cytoplasm and round central nuclei (H and E, ×400)
Scrotal ultrasonography can be useful in differentiating leydig cell hyperplasia from leydig cell tumor by the lesions’ echogenicity. In leydig cell hyperplasia it is a mix of hypo and hyperechoic lesions while in leydig cell tumor it is either homogeneously hypoechoic, or of mixed echogenicity. The more the hyperechogenic lesions in the testes the more necessary to do testicular excisional biopsy with frozen- section analysis [6].
Grossly TARTs are often bilateral while leydig cell tumors are unilateral. Both are brown in color due to lipofuscin pigment. Histopathologically, TARTs are confined to testicular hilum in most cases while leydig cell tumor are located in testicular interstitium. TARTs appear as large polygonal cells with abundant eosinophilic cytoplasm arranged in strands or cords separated by fibrous septae. Features such as lack of cytological atypia, low mitotic activity, dense fibrous septae, lymphoid aggregates are present in TARTs. Reinke crystals are confirmatory of leydig cell tumors, yet present in only 25–40% of cases and absent in TARTs. Immunohistochemically, strong positivity for CD56 as well as strong reactivity for synaptophysin are present in TARTs. Negative reactivity for CD56, and synaptophysin are seen in leydig cell tumors [7].
It is also important to differentiate between leydig cell hyperplasia and leydig cell tumor. Leydig cell tumor is solitary, whereas leydig cell hyperplasia is characteristically multifocal. In addition, leydig cell tumor is larger (more than 0.5cm in diameter), whereas the foci of leydig cell hyperplasia are smaller (less than 0.5cm in diameter). Leydig cell tumor is more likely to be diagnosed with the presence of the distinctive immunopanel (inhibin, calretinin, and Melan-A) also with the identification of Reinke crystals (in some cases) and lipofuscin pigment [8].
In our patient, the clinical examination and the gross appearance of the testes confirmed the multifocality of the lesions favoring the dianosis of leydig cell hyperplasia. The scrotal ultrasound showed that the lesions were multiple and not invasive suggesting again the diagnosis of leydig cell hyperplasia although the report confirmed the presence of TARTs. The scrotal MRI showed features highly suggestive of invasive leydig cell tumor, in agreement to the more confirmative histopathological results that showed the exact features of leydig cell tumor.
Conclusion
This case highlights on the importance of undergoing scrotal ultrasonography, scrotal MRI and histopathological examination for any testicular masses in lately diagnosed congenital adrenal hyperplasia patients.
Conflict of Interest
No conflict of interest
References:
- Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(12):5721-5728.
- Holm M, Meyts ER-D, Andersson A-M, Skakkebæk NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. Journal of Pathology. 2003;199(3):378-386.
- Güven A, Nurcan Cebeci A, Hancili S. Gonadotropin releasing hormone analog treatment in children with congenital adrenal hyperplasia complicated by central precocious puberty. Hormones (Athens).2015;14(2):265-271.
- Wang Z, Yang S, Shi H, Du H, Xue L, Wang L, et al. Histopathological and immunophenotypic features of testicular tumour of the adrenogenital syndrome. Histopathology. 2011;58:1013-1108.
- Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:209-220.
- Maizlin ZV, Belenky A, Kunichezky M, Sandbank J, Strauss S. Leydig cell tumors of the testis: gray scale and color Doppler sonographic appearance. J Ultrasound Med. 2004;23:959-964.
- Tanaka Y, Carney JA, Ijiri R, et al. Utility of immunostaining for S-100 protein subunits in gonadal sex cord-stromal tumors with emphasis on the large- cell calcifying Sertoli cell tumor of the testis. Hum Pathol. 2002;33:285-289.
- Hribar KP, Warner NE, Sherrod AE. Cytologic identification of Reinke crystalloids in scrapings and imprints of fresh testicular tumors: a simple and rapid technique for intraoperative use. Arch Pathol Lab Med. 2005;129:e65-e66.

