The Effect of Coinfection on the Nutritional Status of Young Children in rural communities of Osun State, Southwest Nigeria
Odoemene Simon Nnayere1,4,*, Oluwole, Akinola Stephen2, Mogaji Hammed Oladeji3, Omitola Olaitan Olumide4, Bayegun Adedotun Ayodeji4, Adegbola Victoria Mosebolatan5, Ojo David Ajibola6, Samwobo Sammy Olufemi4 and Ekpo Uwem Friday4
1Department of Basic Science, Adeleke University Ede, Osun State, Nigeria
2The COUNTDOWN Project Department of Neglected Tropical Diseases, Sightsavers, Nigeria Country Office, Kaduna, Nigeria
3Department of Animal and Environmental Biology, Federal University Oye, Ekiti State, Nigeria
4Department of Pure and Applied Zoology Federal University of Agriculture, Abeokuta, Nigeria
5Department of Science Laboratory Technology, Federal Polytechnics Ede, Nigeria
6Department of Microbiology Federal University of Agriculture, Abeokuta, Nigeria
Received Date: 03/04/2024; Published Date: 22/10/2024
*Corresponding author: Odoemene Simon Nnayere, Department of Basic Science, Adeleke University Ede, Osun State, Nigeria
Abstract
Background: Coinfection of parasite is a common infection in the developing countries of Sub-Sahara Africa. However, there is little or no data on coinfection of parasites and its effect on infants/pre-school aged children nutritional status. A survey was undertaken in Egbedore LGA, Osun State, Nigeria, to determine the prevalence of coinfection of parasites and its impact on the nutrition of pre-school aged children in selected rural communities of Osun State.
Fresh stool and blood samples were randomly collected from the participants and processed for helminths and protozoan parasites. The nutritional status of preschoolers was assessed by anthropometric data of weight, height, sex, and age. Mothers/caregivers were interviewed using a structured questionnaire to obtain demographic data of infants and preschoolers and to document their Knowledge, Attitude, and Practices on coinfection of parasites. Data obtained from the questionnaire were analyzed using Epidata version 3.1, while parasitological data were analyzed using Statistical Package for Social Sciences (version 20.0). Descriptive statistics were computed for demographic data, and an association was tested using bivariate analysis at 95% confidence level while significance was set at p < 0.05. Nutritional status was computed as Z-Score; as Weight for Age, Height for Age, Weight for Height, and Body Mass Index for Age, representing underweight, stunting, wasting, and thinness, respectively.
Results: An overall prevalence of 35.54%, 16.18%, and 1.99% for double, triple, and quadruple coinfection of parasite were recorded respectively in the respondents. Although female participants were more infected than males, but no significant difference (𝑃> 0.05) was observed. Significantly (p<0.05), preschoolers (65.93 %) harbor more coinfections than infants (34.07%). Plasmodium falciparum and Ascaris lumbricoides (30.12%), P. falciparum, Ascaris. lumbricoides, and Trichuris trichiura (14.81%) were the highest double and triple coinfecting parasites respectively. The overall prevalence of 34.03%, 23.53%, 24.79%, and 17.65% for underweight, stunting, wasting, and thinness were recorded as nutritional status indicators. No significant difference (p>0.05) was observed in malnutrition among the age groups, but females were significantly (p<0.001) more malnourished for stunting and wasting than males. Malnutrition indices were significantly associated (p=0.001) with triple parasitic infections.
Conclusion: This study showed that coinfection of parasites is of public health and burden in the tropics because of its association with malnutrition in infants and preschool aged children.
Keywords: Coinfection; Infants and Preschool aged children; Prevalence and Nutritional status
Background
Coinfection of parasites is common in the tropics and less developed areas of the world where children/adult are exposed to several parasitic infections at the same time [1], millions of people (mostly preschoolers) are the most at risk of parasite coinfections such as malaria and Neglected Tropical diseases (NTDs) mostly in Sub-Saharah Africa (SSA) and other developing nations [2-4]. Many researchers have documented the association between single parasitic infection with anemia and nutritional health in children [2-5]. According to [6-9] preschoolers infected with more than one parasite simultaneously will be physical unfit, will have low cognitive development, and this will lead to absent from schools, which causes reduced learning and low academic achievements. Coinfections of parasites can also increase higher susceptibility to other infectious diseases agents [7,10,11,12].
Currently 14 NTDs are endemic in Nigeria, making it the highest endemic region in the globe [13]. Malaria infection is endemic in the country, children under five years of age are the most vulnerable group [13]. Malnutrition is a significant public health issue in SSA because it affects the growth and development, thus leading to physical and mental impairment [14].
Scientist have identified malnutrition due to coinfection of parasites as one of the major cause low cognitive functioning in preschoolers [2-4,15,16]. It affects the physical, mental, social wellbeing and development of children. Malnutrition has led to the death of over half of children born in many developing countries of the world [17,18]. In 2019, it was estimated that 149 million children under the age of five were stunted, 40 million were underweight while 49 million were wasted [19].
Studies related to coinfection of parasites in Nigeria have focused mainly on investigating the prevalence and intensity of infections [20,21,22] on the host, with little or no data reported on its effect on host's nutrition, especially infants and preschool-aged children. Therefore, this study was undertaken to survey the coinfection of parasites and its effect on the nutritional status of Infants and Preschool-Aged Children in (IPSAC) Egbedore District, Osun state, Nigeria.
Methods
Study area
Egbedore is a Local Government Area (LGA) in Osun State. The secretariat of the LGA is located at Awo (Lat 7°46′00′′N Long 4°24′00′′E). According to the 2006 census, the land area was 270 km2, with a projected population of about 101,900 in 2016 of which 21, 231 were preschoolers. The inhabitants of the area are the Yoruba tribe, which mainly engages in small-scale agriculture and livestock farming. The LGA consists of seven districts, with rural communities attached to the districts.
Ethical Approval
Approval for the study was obtained from Osun State Health Research Committee (OSHREC) Ministry of Health (Ref. Number: OSHREC/PRS/569T/66). Parent of the young children also gave their consent after they were sensitized and mobilized.
Study design and sampling procedures
Sampling of households was done in the ten communities selected for this stud, infants and preschool-aged children were recruited randomly with the study area [23]. Demographic characteristics of the study participants were retrieved using a structured questionnaire. Blood, stool and anthropometric (weight, height, age) data were analyzed.
Data collection and laboratory methods
Stool samples: A small amount of stool (1-2 grams) is emulsified in a tube containing SAF solution for preservation and fixative of the stool, the mixture is then strained through gauze or a tea strainer to remove large particles and debris. The strained suspension is then transferred into a centrifuge tube and centrifuged at 2000rpm for 1min. After centrifugation, the supernatant is carefully decanted, leaving behind a pellet of concentrated material at the bottom of the tube. The tube is vigorously shaken to mix the sediment with ether, and then centrifuged again. The supernatant is carefully decanted, leaving behind a concentrated sediment at the bottom of the tube. A drop of the sediment is placed on a microscope slide, covered with a coverslip, and examined under a microscope for the presence of parasites, ova, and cysts.
Blood samples: Lancet was used to prick the patient's fingertip after cleaning with alcohol swab, 10μl of blood collected and examined for the presence of malaria parasite using using CareStartsTM Malaria pf (HRP2).
Anthropometric Measurement: Anthropometric indices such as weight, height, and age were recorded for each child providing stool and blood samples. Weight was measured in kilograms (Kg) using a domestic HAMSON bathroom weighing scale. The weights of infants (0-12month) were measured by weighing them together with their mother/caregiver and the mother’s weight is deducted from the total weight measured. Height was measured using the builders' tape, and age of the children was collected from hospital records and their mothers or caregivers.
Questionnaire administration: Information on knowledge, attitude, and practice (KAP) of caregivers on parasitic coinfections, control, prevention, and treatments were documented using pretest questionnaire. The same research instrument was used to source information on demographic data of caregivers, preschoolers, and sanitary and hygiene conditions of households selected for the study.
Anthropometric/Nutritional Analysis: Nutritional analyses were carried out using IBM Height-for-Age (HAZ), Weight for- Age (WAZ), Weight for Height (WHZ), and BMI-for-Age (BMZ) obtained from weight, height, and age data according to WHO Child Growth Standard using the WHO Child Growth Standards SPSS Syntax File for SPSS (2007) for children age 0–5 years and the WHO Reference 2007 SPSS macro package (2008) for the data for children of age 6. Children whose HAZ, WAZ, and BMZ were above −2 S.D. scores were considered normal, and below −2 SD and −3 S.D. scores were considered moderately malnourished and severely malnourished, respectively.
Data Analysis: All the data obtained from the survey were entered into Microsoft Excel and analyzed using IBM Statistical Package for Social Sciences (SPSS) version 20.0. Descriptive statistics were computed for demographic data.
Results
Demographic Characterization
One thousand and sixty (1060) preschoolers were recruited and examined for this survey, of which 48.68% were males and 51.32% were females. Preschoolers within age bracket 13-24months old (19.91%) were the highest recruit for this study as shown in table 1 below.
Table 1: Demographic Characteristic of the study group in the communities.
Categories of Coinfections
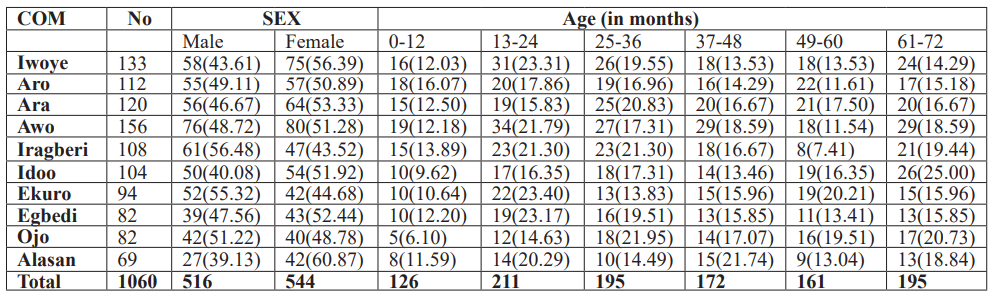
A total of 405 preschoolers had coinfection of which 66.17% had double infection, 30.17% had triple infection while 3.70% respondents had more than three parasite infections simultaneously as seen in figure 1. Plasmodium falciparum (pf), Ascaris lumbricoides (As), Trichuris trichiura (Tt), Hookworm (Hw) and Entamoeba histolytica (Eh) were the common co-infecting parasites according to the survey.
The highest coinfecting parasite were Pf and As (30.12%), followed closely by Pf + As + Tt. (14.81%) and As + Tt. (11.85%) respectively. However, no significant difference (p>0.05, p=0.210) was observed in parasitic coinfections within the communities. Females (52.59%) were mostly infected with coinfection than the males (47.41%), however the result was statistically insignificant (p>0.05). Preschoolers within age bracket 13-24month old (22.96%) had the highest prevalence of coinfection in the study area; but the result showed no significant difference (p>0.05) within the age brackets as seen in figure 3.
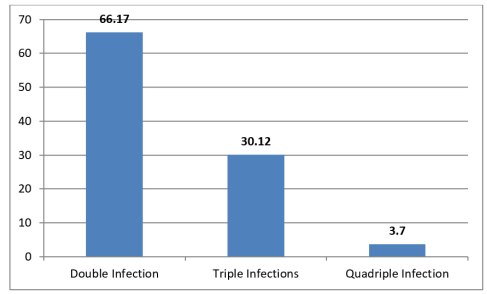
Figure 1: Categories of coinfections in the study area.
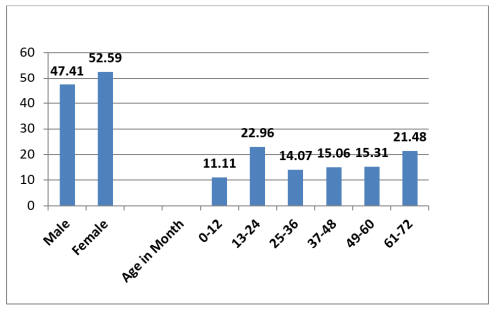
Figure 2: Coinfections by Sex and Age of the surveyed IPSAC.
Prevalence of Malnutrition
The overall prevalence of malnutrition recorded among infants and pre-school aged children in this study was 34.03%, 23.53%, 24.79%, and 17.65% for underweight, stunting, wasting, and thinness conditions, respectively (Table 2). A total of 99(20.80%) of the IPSAC were moderately malnourished for underweight. In comparison, 71(14.92%), 75(15.76%), and 56(11.76%) were moderately malnourished for stunting, wasting, and thinness conditions; however, 63(13.23%), 41(8.61%), 43(9.03%) and 28(5.88%) of the surveyed IPSAC had severe underweight, stunting, wasting and thinness conditions respectively. Female IPSAC were more malnourished than males in this survey with underweight (52.622% versus 49.36%), stunting (56.68% versus 47.32%), wasting (58.47% versus 41.53%), and thinness (51.19% versus 48.81%) for females and males respectively.
Table 2: Nutritional indices (𝑍-score) of the surveyed IPSAC in the study area (n=476).
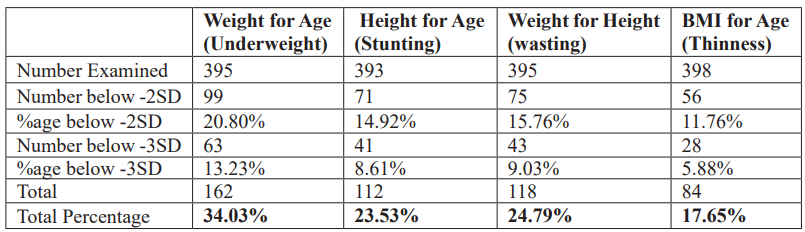
However, there were no significant differences (p>0.05) in malnutrition indices recorded for sex except for stunting and wasting (p<0.05) conditions. Infant and Pre-School Aged Children between age 13-24months 139(29.20%) recorded the highest malnourishment among the age groups. There was no significant difference (p>0.05) observed in nutritional indices among the age categories except for underweight (p>0.000) (Table 3).
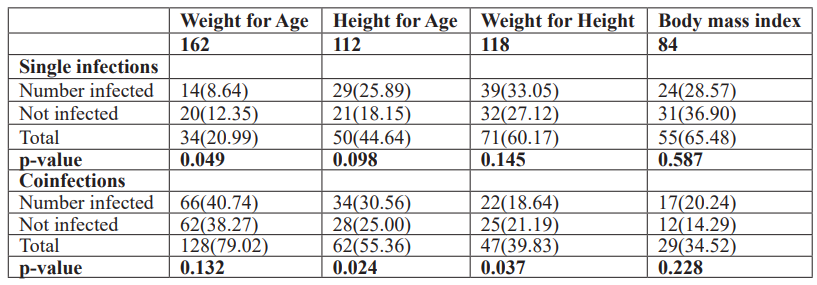
Association between Parasitic coinfection and malnutrition
Most malnourished IPSAC were infected with single parasite and coinfections, with malnutrition prevalence of 8.64% versus 40.74% for underweight, 25.89%versus 30.56% for stunting, 33.05% versus 18.64% for wasting, and 28.57% versus 20.24% for thinness conditions. However, there was no significant difference (𝑃>0.05) observed across the categories. However, IPSAC infected with coinfections was more malnourished for underweight and stunting conditions than IPSAC infected with the single parasite. This study also observed that IPSAC infected with coinfection were more malnourished than non-infected IPSAC in the study area for underweight, stunting, and thinness conditions with 40.74% versus 38.27% 30.56% versus 25.00% and 20.24% versus 14.29%, respectively. However, no significant differences (𝑃>0.05) were observed (Table 4).
Table 3: Nutritional indices (Z-scores) of the IPSAC by sex and age.
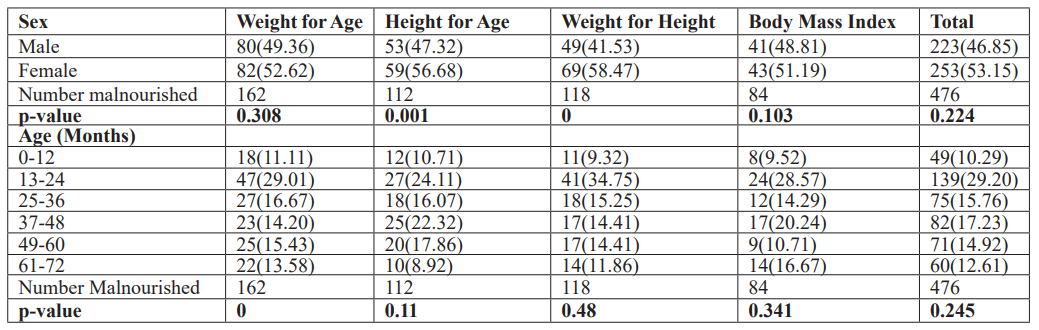
Table 4: Nutritional indices (Z-scores) and parasitic infections among IPSAC.
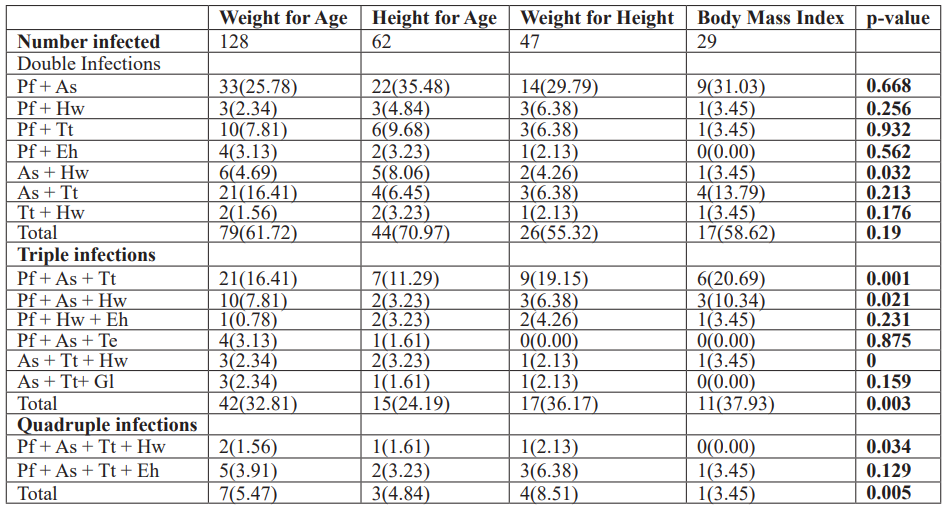
Infant and Pre-scholars infected with P. falciparum and A. lumbricodes simultaneously were malnourished for underweight, stunting, wasting, and thinness condition, but no significant difference (p>0.05) was observed. Triple and quadruple parasitic infections were significantly associated (p<0.05) with malnutrition in this study as IPSAC infected with more than two parasites were more malnourished for underweight, stunting, wasting, and thinness conditions with 32.81% versus 5.47%, 24.19% versus 4.84%, 36.17% versus 8.51% and 37.93% versus 3.45% respectively (Table 5).
Table 5: Nutritional indices (Z-scores) and Parasitic coinfection among IPSAC in the study area.
Discussion
This current study shows an overall prevalence of 34.03%, 23.53%, 24.79%, and 17.65% for underweight, stunting, wasting, and thinness as indicators of nutritional status among the surveyed IPSAC, this is of great public health concern. The prevalence recorded in this study is higher than that reported by [17] in a study conducted in Uganda where 5.3% of the children examined were underweight, 22.5% were stunted, and 18.5% were wasted. Studies conducted in Ethiopia, China, and India reported higher stunting prevalence of 26.5%, 25.6%, and 37% respectively among children [24-26], compared to the stunting prevalence in this current study.
However, the prevalence of 34.03% reported for underweight conditions in this study is lower than the report of [27] in Abeokuta, Nigeria, where 56.6% of pre-school children surveyed were underweight. The prevalence of underweight reported in this study was also low compared to the one reported in other countries. Several studies [26,28,29] done in Indian school children showed an underweight prevalence of 51.7%, 60.9%, and 44%, respectively. The low prevalence of underweight reported in our study may be attributed to the routine deworming activities in the communities surveyed as reported by health workers and parents/caregivers. This was also reported by [30], where it was revealed that regular deworming of children could increase weight among the children.
Infectious parasites recorded in the study are Plasmodium falciparum, hookworm spp, Ascaris lumbricoides, Trichuris trichiura, Entamoeba histolytica, Giardia lamblia, and Taenia spp. The prevalence of coinfection is 53.71% (405/754), which is high compared to a study undertaken by [31] and [32] in coastal Kenya in which the prevalence of 31.80% and 38% of the children examined were infected with coinfection. The predominant double parasitic infections were P. falciparum and A. lumbricoides (16.18%), while P. falciparum, T. trichiura, and A. lumbricoides (7.95%) were the predominant triple parasitic infection recorded in this study. The high prevalence of coinfection recorded in this study is in similitude with the findings of [32] in Côte d'Ivoire, where school children were infected with two or more species concurrently.
The high rate of co-infection in this present study is an indication that parasitic infection is endemic in the study area; this could be due to relevant environmental factors in the study area that favor parasites larva development (climate, adequate soil moisture, warm temperature), low socioeconomic status and poor hygiene condition of the study area which aid transmission of parasites.
Most of the malnourished IPSAC in this study were infected with P. falciparum, A. lumbricoides, T. trichiura, and hookworm or their combinations compared with non-infected. The report also showed that co-infection of malaria and STH infections are essential predictors for malnutrition. Previous studies had found T. trichiura, A. lumbricoides, or their co-infection, all associated with malnutrition [29,32,33].
Though malnutrition is multi-factorial, it is mostly associated with socioeconomic status, where the poor in the society are more likely to be malnourished. Still, this study has also revealed that a right proportion of the malnourished IPSAC in this study area were infected with co-infections of P. falciparum, A. lumbricoides, T. trichiura, and hookworm. The reason for differences in malnutrition indices between this study and other studies may be attributed to differences in parasitic prevalence, intensity, and the study participants' age.
Conclusion
This study shows that parasitic coinfections is prevalent in Egbedore LGA, Osun State Southwest, Nigeria, among infants and preschoolers. It is a significant public health problem because it is significantly associated with malnutrition. Hence there is a need for improved environmental conditions, including adequate water supply, periodic deworming of infants, pre-school, and school-aged children. An integrated control approach of malaria and STH infections among infants and preschool-aged children in endemic areas can play a vital role in improving their nutritional status. This study needs further investigation.
Ethical approval and consent to participate: The Approval for this study was obtained from Osun State Health Research Committee (OSHREC), Ministry of Health (Ref. Number: OSHREC/PRS/569T/66). A notification was sent to the public health director in the LGA and coordinators of the primary health center in each community. They all gave their consent to the study and assisted in the sensitization and mobilization of households. The researcher distributed informed consent forms to mothers/caregivers. The documents were verbally translated to the parents and caregivers in their local language; only mothers/caregivers who consented by signing the consent form were recruited into the study.
Consent for Publications: No data, images or video of an individual is included in this manuscript.
Availability of Data and Material: The dataset during and/or analyzed during the current study is available from the corresponding author on a reasonable request.
Competing Interests: The authors have declared that they have no competing interest in this study.
Funding: No funding was received for this current study.
Authors’ Contributions: OSN conceived, designed, coordinate the study, and prepare the manuscript. OAS and MHO took part in the analysis and interpretation of the work. OOO, AA Band AMV participated in the data collections and statistical analysis; also, they took part in conducting the literature search. SSO ODA and EUF supervised and critically review the paper. All authors read and approve the final draft.
Acknowledgements: The authors wish to thank the Ministry of Health Osun State, Directors of Public Health in the LGA and coordinators of public health centers in each community surveyed, community heads and heads of households for their support and for allowing us to carry out this current study. Our sincere appreciation goes to the infants and preschoolers who participated in the study.
References
- Bisanzio D, Mutuku F, Bustinduy AL, Mungai PL, Muchiri EM, King CH, et al. Cross-sectional study of the burden of vector-borne and soil-transmitted polyparasitism in rural communities of Coast Province, Kenya. PLoS Neglected Tropical Diseases, 2014; 8: e2992.
- Snow RW, Guera CA, Noor AM, Myint HY, Hay HI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature, 2005; 434: 214–217.
- Walker CL, Rudan I, Liu I, Nair H, Theodoratou E, Bhutta ZA, et al. Campbell H, Black R.E: Global burden of childhood pneumonia and diarrhea. Lancet, 2013; 381: 1405-1416.
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites and Vectors, 2014; 7: 37.
- Stoltzfus RJ, Chway HM, Montresor A, Tielsch JM, Jape JK, Albonico M, et al. Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite, and anemia in Zanzibari preschool children. Journal of Nutrition, 2004; 134: 348–356.
- Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology, 2008; 135: 783–794.
- Molyneux DH, Hotez PJ, Fenwick A. ‘‘Rapid-impact interventions’’: How a policy of integrated control for Africa’s neglected tropical diseases could benefit the poor. PLoS Med, 2005; 2(11): e336.
- Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL. Helminth infection and cognitive impairment among Filipino children. America Journal of Tropical Medicine and Hygiene, 2005; 72(5): 540–54
- Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. Journal of Parasitol, 2002; 88(1): 55–5
- Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M. Increased malaria attacks among children presenting concomitant infections by S. mansoni in Senegal. Malaria Journal, 2004; 3: 43.
- Hillier SD, Booth M, Muhangi L, Nkurunziza P, Khihembo M, Kakande M. Plasmodium falciparum and helminth co-infections in semi urban population of pregnant women in Uganda. Journal of Infectious Diseases, 2008; 198: 920–92
- Amosu AM, Degun AM, Atulomah NOS, Olanrewaju MF. A Study of the Nutritional Status of Under-5 Children of Low-Income Earners in a South-Western Nigerian Community. Journal of Biological Sciences. 2011; 3(6): 578 – 585.
- World Malaria Report, 2019.
- Laditan AA. Nutrition and physical growth in children Nigeria. Journal of Nutritional Science, 1983; 4: 5 – 10.
- Ake-Teno O, Ekou FK, Tetchi EO, Koffi KB, Oussou KR, Kpebo DOD, et al. Determinants of Malnutrition in Under-five Children followed at the National Institute of Public Health Cote d’Ivoire. Africa Med. Black, 2011; 58(2): 93-99.
- Rai SK, Hirai K, Abe A, Ohno Y. “Infectious diseases and malnutrition status in Nepal: an overview,” Malaysian Journal of Nutrition, 2002; 8(2): pp. 191–200.
- Lwanga F, Kirunda BE, Orach CG. “Intestinal helminth infections and nutritional status of children attending primary schools in Wakiso district, Central Uganda,” International Journal of Environmental Research and Public Health, 2012; 9(8): pp. 2910–2921.
- Del Rosso JM. School Feeding Programs: Improving Effectiveness and Increasing the Benefit to Education. The Partnership for Child Development Publication, Oxford, UK, 1999.
- UNICEF/WHO/The World Bank Group joint child malnutrition estimates-levels and trends in child malnutrition 2019 edition, WHO/NMH/NHD/19.20.
- Chopra M. “Mass de-worming in Ugandan children,” British Medical Journal, 2006; 333: no. 7559.
- United Nation Children Fund. World Health Organization and World Bank. Joint Child Malnutrition Estimates. New York/Geneva/Washington DC. UNICEF, WHO and World Bank, 2019; 9-10.
- Ijagbone IF, Olagunju TF. “Intestinal helminths parasites in school children in Iragbiji, Boripe local government, Osun State, Nigeria,” African Journal of Biomedical Research, 2006; 9(1): pp. 63–65.
- Ekpo UF, Alabi OM, Oluwole AS, Sam-Wobo SO. “Schistosoma haematobium infections in preschool children from two rural communities in Ijebu East, south-western Nigeria,” Journal of Helminthology, 2012; 86(3): pp. 323–328.
- Mulugeta A, Hagos F, Stoecker B. “Nutritional status of adolescent girls from rural communities of Tigray, Northern Ethiopia,” Ethiopian Journal of Health Development, 2009; 23(1): pp. 5–11.
- Shang Y, Tang L-H, Zhou S-S, Chen Y-D, Yang Y-C, Lin S-X. “Stunting and soil-transmitted-helminth infections among school-age pupils in rural areas of southern China,” Parasites and Vectors, 2010; 3: 97.
- Osei A, Houser R, Bulusu S, Joshi T, Hamer D. “Nutritional status of primary schoolchildren in Garhwali Himalayan villages of India,” Food and Nutrition Bulletin, 2010; 31(2): pp. 221–223.
- Omitola OO, Mogaji OH, Oluwole AS, Adeniran AA, Alabi OM, Ekpo UF. Geohelminth infection and Nutritional status of Pre-school Aged Children in periurban settlement of Ogun State. Scientifica, 2016; 2016: 7897351.
- Medhi GK, Barua A, Mahanta J. “Growth and nutritional status of school-age children (6–14 years) of tea garden workers of Assam,” Journal of Human Ecology, 2006; 19: pp. 83–85.
- Nitish M, Jaydip S. Prevalence of under-nutrition among children (5–12 years) belonging to three communities residing in a similar habitat in North Bengal. Annals Human Biolology, 2010; 37: 199 217.
- Harold A, Konde-Lule J, Sebuliba I, Bundy D, Hall A. Effect on weight gain of routinely giving albendazole to preschool children during child health days in Uganda. Cluster randomized controlled trial. Biomedical Journal, 2006. doi:10.1136/bmj.38877.393530.
- Florey LS. Epidemiology of polyparasitism in coastal Kenya: Determinants, interactions and health effects of Plasmodium species and Schistosoma haematobium infections. Case Western Reserve University, 2009.
- Hürlimann E, Yapi RB, Houngbedji CA, Schmidlin T, Kouadio BA, Silué KD. The epidemiology of polyparasitism and implications for morbidity in two rural communities of Côte d’Ivoire. Parasite and Vectors, 2014; 7: 81.
- Quihui-Cota L, Valencia ME, Crompton DWT, Philips S, Hangan P, Diaz- Camacho SP, et al. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Transactions of the Royal Society Tropical Medicine Hygiene, 2004; 98: 653-659.

