Clinical Pharmacology of Diflunisal
Gian Maria Pacifici*
Professor of Pharmacology, Via Sant’Andrea 32, Italy
Received Date: 29/07/2024; Published Date: 17/10/2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56127 Pisa, Italy
Abstract
Diflunisal is a difluorophenyl derivative of salicylic acid, is not converted to salicylic acid, is a competitive inhibitor of cyclooxygenase, and is a potent anti-inflammatory drug. Diflunisal has been used as an analgesic in treatment of osteoarthritis and musculoskeletal strains or sprains. Diflunisal is rapidly absorbed, partially from the stomach, but mostly from the small intestine, the peak concentration is reached in about 2 to 3 hours, and diflunisal is distributed throughout most body tissues and transcellular fluids. In adults, the oral dose of diflunisal is 250 to 500 mg twice-daily or thrice-daily, and diflunisal is sulfated and glucuronidated. The efficacy and safely of diflunisal, the treatment with diflunisal, and the trials conducted with diflunisal have been reviewed. The pharmacokinetics of diflunisal have been studied in healthy volunteers and in patients with moderate renal insufficiency, in patients with preterminal renal insufficiency, and in patients with terminal renal insufficiency and the mean elimination half-life of diflunisal is 10.8, 22.4, 59.6, and 115 hours, respectively. The metabolism of diflunisal has been reviewed and diflunisal is converted into diflunisal sulfate, diflunisal acyl glucuronide, and into diflunisal phenolic glucuronide. The interaction of diflunisal with drugs and the toxicity induced by diflunisal have been reviewed. The aim of this study is to review the efficacy and safely of diflunisal, the treatment with diflunisal, and the trials conducted with diflunisal. In addition, the metabolism of diflunisal, the interaction of diflunisal with drugs, and the toxicity induced by diflunisal have been reviewed.
Keywords: Diflunisal; Drug-interaction; Efficacy-safely; Metabolism; Pharmacokinetics; Toxicity; Treatment; Trials
Introduction
Therapeutic uses of diflunisal
Diflunisal is a difluorophenyl derivative of salicylic acid that is not converted to salicylic acid in-vivo. It is a competitive inhibitor of cyclooxygenase and a potent anti-inflammatory drug but is largely devoid of antipyretic effects, perhaps because of poor penetration into the cerebrospinal system. The drug has been used primarily as an analgesic in the treatment of osteoarthritis and musculoskeletal strains or sprains, in these circumstances; it is about three to four times more active than aspirin. Diflunisal may produce fewer auditory adverse-effects and appears to cause fewer and less-intense gastrointestinal and antiplatelet effects than aspirin [1].
Absorption, distribution, metabolism, and elimination of diflunisal
Orally ingested diflunisal is absorbed rapidly, partially from the stomach, but mostly from the upper small intestine. The peak plasma concentration is reached about 2 to 3 hours. The rate of absorption is determined by disintegration and dissolution-rates of the tablets, by the pH at the mucosal surface, and by the gastric emptying time. Even though diflunisal is more ionized as the pH is increased, a rise in pH also increases the solubility of diflunisal and thus dissolution of the tablets. The presence of food delays absorption of diflunisal. After absorption, diflunisal is distributed throughout most body tissues and transcellular fluids, primarily by pH-dependent processes. Diflunisal is transported actively out of the cerebrospinal fluid across the choroid plexus. Diflunisal is bound to plasma protein for 99% especially to albumin, the proportion of the total that is bound declines as plasma concentration increases. Diflunisal is conjugated with sulphate and is glucuronidated and the oral dose of diflunisal is 250 to 500 mg twice-daily or thrice-daily in adults and is 10 to 15 mg/kg 6 times-daily in children aged 12 years or older. In adults, the elimination half-life of diflunisal is 8 to 12 hours.

Figure 1: Molecular structure of diflunisal (molecular weight = 250.201 grams/mole).
Literature search
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “diflunisal efficacy, safely”, “diflunisal treatment”, “diflunisal trials”, “diflunisal pharmacokinetics”, “diflunisal metabolism, “diflunisal drug interactions”, and “diflunisal toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Results
Efficacy and safely of diflunisal
Diflunisal was administered at the dose of 250 mg twice-daily and effectively and safely treated patients with moderate to severe neuropathy and cardiomyopathy [2]. Diflunisal was administered at the daily dose of 500 mg to patients with familial amyloid polyneuropathy and diflunisal effectively and safely treated these patents [3]. A single dose of 1,000 mg of diflunisal was administered to 20 patients undergoing the removal of impacted third molars. Treatment with diflunisal 30 minutes after completion of surgery effectively and safely controlled postsurgical pain [4]. Diflunisal was administered at the daily dose of 125 mg to 33 patients with limited postsurgical pain, at the daily dose of 250 mg to 30 patients with moderate postoperative pain, and at the daily dose of 500 mg to 30 patients with high postoperative pain and diflunisal effectively and safely treated postsurgical pain in all patients [5]. Oral ointment of 2% of diflunisal was administered to 8 patients with painful oral mucous diseases. Diflunisal was found to be significantly (P-value = 0.014) more effective than 2% aspirin oral ointment. A 2% diflunisal oral ointment is a clinically useful analgesic for painful oral lesions [6].
Treatment with diflunisal
Diflunisal was administered at the daily dose of 500 mg to 35 patients with transthyretin amyloid cardiomyopathy and diflunisal improved survival-rate and overall stability in clinical and echocardiographic markers of disease [7]. Diflunisal was administered at the daily dose of 500 mg to 23 patients with transthyretin amyloidosis and to 13 patients with hereditary transthyretin amyloidosis subtype and diflunisal effectively treated all patients [8]. Diflunisal was administered at the dose of 500 mg twice-daily to a patient aged 61 years with amyloid disease allergic to ibuprofen and diflunisal treated the patient [9]. A total of 81 patients with transthyretin amyloidosis cardiomyopathy received diflunisal at the dose of 250 mg twice-daily and this treatment cured all patents [10]. Three doses of 500 mg of diflunisal were given to 30 patients undergoing the removal of impacted mandibular third molar and 30 patients received placebo. Diflunisal extinguished the pain more effectively than placebo (P-value < 0.0001). Diflunisal, compared to placebo, is a highly effective and well-tolerated analgesic in the treatment of postoperative pain following surgical removal of impacted mandibular third molars [11]. Diflunisal was administered at the daily dose of 500 to 750 mg to patients with osteoarthritis pain and was more efficacious than ibuprofen administered at the daily dose of 800 to 1,200 mg. Diflunisal had a longer duration of action than ibuprofen and required only once-daily administration [12]. Thirty-three patients with active rheumatoid arthritis received either diflunisal at the dose of 500 mg twice-daily or naproxen at the dose of 375 mg twice-daily. Both drugs resulted in marked reduction in the number of swollen, tender, and had comparable improvement of disease. Diflunisal and naproxen were equally effective and well-tolerated in patients with active rheumatoid arthritis [13]. Fifty patients with osteoarthritis received either diflunisal at the daily dose of 500 mg or aspirin at the daily dose of 500 mg. Diflunisal was better tolerated, caused fewer adverse-effects and less increase in cell and lysosomal enzyme excretion by the kidney than aspirin [14].
Trials conducted with diflunisal
A single-arm, open-label trial determined the safely and efficacy of diflunisal administered at the dose of 250 mg twice-daily to 13 patients with confirmed wild-type or mutant transthyretin cardiac amyloidosis and diflunisal was well-tolerated and treated the patients [15]. A randomized, clinical trial was conducted in 64 patients with familial amyloid polyneuropathy who received diflunisal at the dose of 250 mg twice-daily and in 66 patients who received placebo and both diflunisal and placebo were administered for 2 years. Diflunisal was more effective than placebo (P-value < 0.05) in the progression of neurological impairment and preserved the quality of life [16]. A randomized, placebo-controlled, clinical trial was conducted in 130 patients with familial amyloidotic polyneuropathy and diflunisal controls the neurologic disease progression and effectively treated the patients [17]. An international, randomized, placebo-controlled trial was conducted to determine the effect of diflunisal on the progression of neurologic disease in patients with active familial amyloidotic polyneuropathy and diflunisal was well-tolerated and treated the patients [18]. In a 12-week, double-blind, controlled trial diflunisal was compared to naproxen in treatment of patients with rheumatoid arthritis. Both diflunisal and naproxen were administered at the daily dose of 500 mg. At week 12 of treatment, the improvement of rheumatoid arthritis was observed with both drugs. Diflunisal cased fewer adverse-effects than naproxen and the adverse-effects were related to the gastrointestinal-tract and were not serious. Diflunisal and naproxen are effective analgesic agents in treatment of patients with rheumatoid arthritis and diflunisal is better tolerated [19]. Two double-blind, inter-group trials were conducted with diflunisal in 30 patients with osteoarthritis of the knees and hips who received either diflunisal, naproxen, or aspirin at the daily dose of 500 mg. Aspirin was administered for 12 weeks and diflunisal and naproxen were administered for 8 weeks. Diflunisal was somewhat better than aspirin and naproxen in terms of both effectiveness and tolerance and effectively treated the patients with osteoarthritis of the knees and hips [20].
Pharmacokinetics of diflunisal
De Schepper et al. [21] studied the pharmacokinetics of diflunisal in healthy volunteers and in patients with renal insufficiency. Table 1 summarizes the pharmacokinetic parameters of diflunisal which have been obtained in healthy volunteers and in patients with renal insufficiency.
Table 1: Pharmacokinetic parameters of diflunisal which have been obtained in 5 healthy volunteers and in 17 patients with renal insufficiency. A single oral dose of diflunisal of 500 mg was administered to healthy volunteers and to patients. Values are the mean+SD, by De Schepper et al. [21].
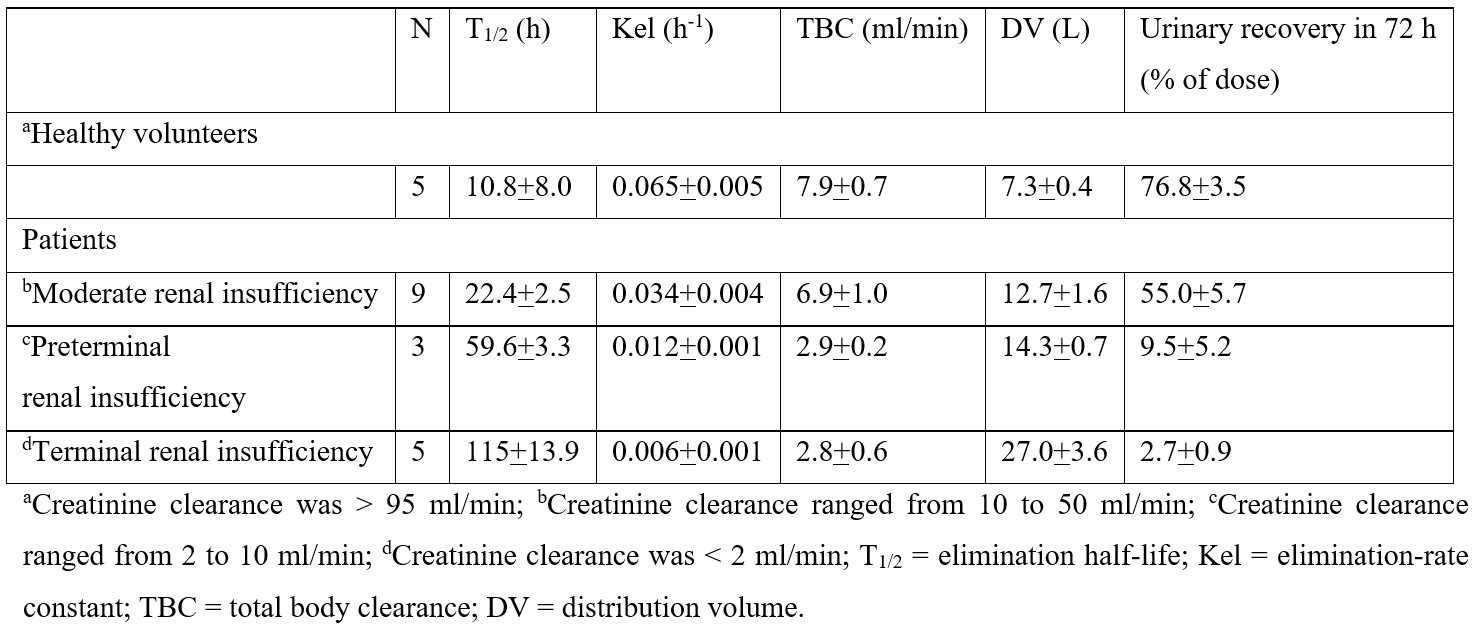
This table shows that the elimination half-life of diflunisal is shorter in healthy volunteers than in patients and increases with the degree of renal impairment. Consisting with these results, the elimination-rate constant of diflunisal is higher in healthy volunteers than in patients and decreases with the degree of renal impairment, the total body clearance of diflunisal is higher in healthy volunteers than in patients and decreases with the degree of renal impairment, the distribution volume of diflunisal is lower in healthy volunteers than in patients and increases with the degree of renal impairment, and the recovery of diflunisal in the urine is higher in healthy volunteers than in patients and decreases with the degree of renal impairment.
Diflunisal was rapidly absorbed and reached the peak plasma concentration of 60 to 80 µg/ml within 2 hours after the dose in healthy volunteers and in patients with moderate and with preterminal renal insufficiency. The peak plasma concentration of diflunisal of 30 µg/ml was reached within 4 hours after the dose in patients with terminal renal insufficiency possibly due to the decreased oral availability. Total diflunisal glucuronides (acyl and phenolic glucuronides) rapidly appeared in plasma reaching maximal concentrations of 8 to 10 µg/ml 2 to 4 hours after the dose in healthy volunteers and in patients with moderate and with preterminal renal insufficiency and appeared in plasma approximately 6 hours after the dose in patients with terminal renal insufficiency. The elimination half-life of total diflunisal glucuronides was 14.8 hours in healthy volunteers, 32.7 hours in patients with moderate renal insufficiency, 84.4 hours in patients with preterminal renal insufficiency, and 219 hours in patients with terminal renal insufficiency. These results indicate that the pharmacokinetic parameters of diflunisal are altered in patients with renal insufficiency.
Metabolism of diflunisal
Loewen et al. [22] studied the metabolism of diflunisal in 6 healthy male volunteers aged 18 to 24 years and weighing 73.6+6.8 kg who received a single oral dose of diflunisal of 100, 250, 500, 750, or 1,000 mg and diflunisal was sulfated and glucuronidated. The urinary recovery of diflunisal and its metabolites ranged from 78.9+11.9% to 91.5+18.7% of the administered dose. The urinary recovery of diflunisal sulfate increased with the dose and ranged from 9.3+3.7% to 18.1+4.8%. The urinary recovery of diflunisal phenolic glucuronide was unaffected by the dose and ranged from 30.6+3.8% to 40.6+6.6% and the urinary recovery of diflunisal acyl glucuronide significantly decreased from the dose and ranged from 52.3+4.6% to 40.2+3.4%. These results indicate that the metabolism of diflunisal consists in sulfation and glucuronidation and the glucuronidation of diflunisal is predominant over the sulfation of diflunisal.
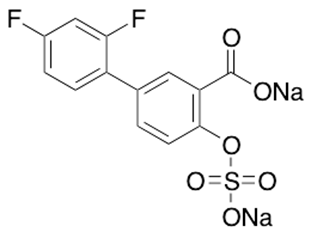
Figure 2: Molecular structure of diflunisal sulfate disodium salt (molecular weight = 374.224 grams/mole).
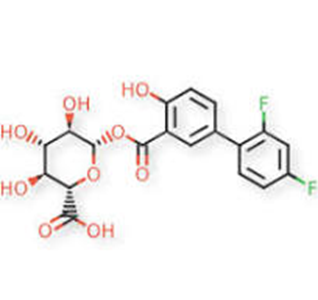
Figure 3: Molecular structure of diflunisal acyl glucuronide (molecular weight = 426.33 grams/mole).
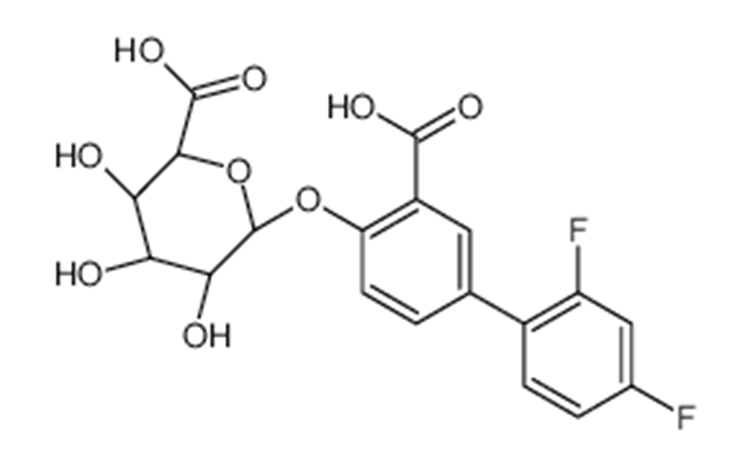
Figure 4: Molecular structure of diflunisal phenolic glucuronide (molecular weight = 426.322 grams/mole).
Table 2 shows the urinary recovery of diflunisal and metabolites, diflunisal, diflunisal sulfate, diflunisal phenolic glucuronide, and diflunisal acyl glucuronide.
Table 2: Urinary recovery (% of administered dose) of diflunisal and metabolites, diflunisal, diflunisal sulfate, diflunisal phenolic glucuronide, and diflunisal acyl glucuronide. Five single oral doses of diflunisal were administered to 6 healthy male volunteers. Values are the mean+SD, by Loewen et al. [22].
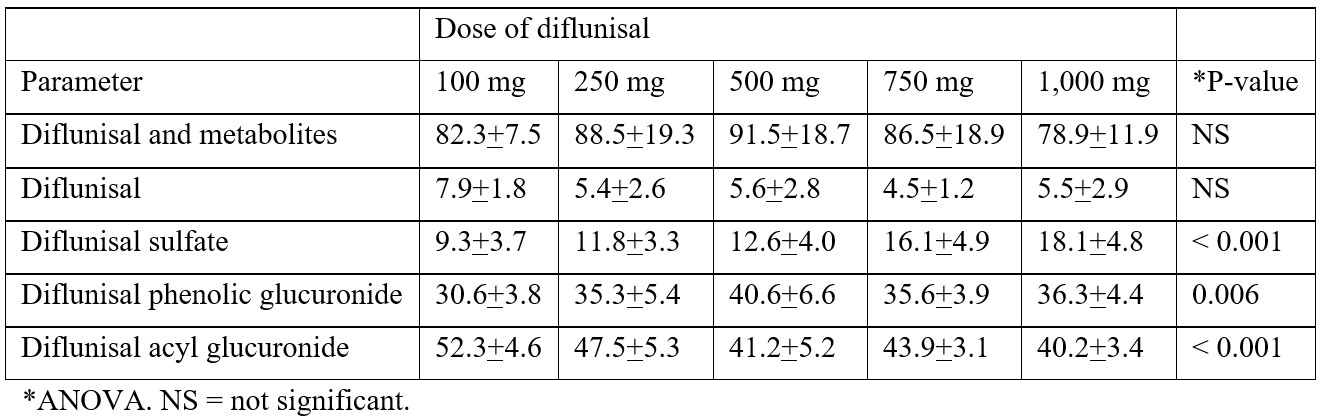
This table shows that most of diflunisal and its metabolites are recovered in the urine and the urinary excretion of diflunisal and its metabolites does not vary with the diflunisal dose. Diflunisal is extensively metabolized and the recovery of diflunisal in the urine is less than 10%. The urinary excretion of diflunisal sulfate increases with the diflunisal dose whereas the urinary excretion of diflunisal phenolic glucuronide and diflunisal acyl glucuronide does not increase with the dose. The urinary excretion of diflunisal phenolic glucuronide is similar to the urinary excretion of diflunisal acyl glucuronide and the glucuronidation of diflunisal is predominant over the sulfation of diflunisal. Herman et al. [23] studied the effect of contraceptive consumption and cigarette smoking on the metabolism of diflunisal. The urinary excretion of diflunisal sulfate, diffusional acyl glucuronide, and diflunisal phenolic glucuronide was assessed in 110 healthy volunteers who received a single dose of 50 mg of diflunisal. Females using oestrogen-containing oral contraceptives excreted 50% less diflunisal sulfate and 20% more diflunisal acyl glucuronide than non-users. The urinary recovery of diflunisal sulfate was reduced by about 30% in cigarette smokers. The metabolism of diflunisal is altered by cigarette smoking and by the consumption of oral contraceptives. Macdonald et al. [24] studied the effects of liver cirrhosis on the pharmacokinetics and metabolism of diflunisal. A single dose of 250 mg of diflunisal was administered to 5 patients with liver cirrhosis and to 5 healthy volunteers. The total body clearance of diflunisal was 10.2 ml/min in healthy volunteers and 10.9 ml/min cirrhotic patients. The percent unbound fraction of diflunisal in plasma was 0.089 and 0.147 (P-value < 0.05) in healthy volunteers and in cirrhotic patients, respectively. The total body clearance of unbound diflunisal was 11.5 and 7.41 ml/min (P-value < 0.05) in healthy volunteers and in cirrhotic patients, respectively. In cirrhotic patients, the unbound partial clearances of phenolic and acyl glucuronides were both significantly reduced, by approximately 38%. The unbound partial clearance to the sulphate conjugate was not significantly affected by cirrhosis. These results indicate that both the phenolic and acyl glucuronidation pathways of diflunisal are susceptible to the effects of liver cirrhosis.
Interaction of diflunisal with drugs
Diflunisal was administered at the dose of 500 mg twice-daily for 2 weeks and increased the percentage of unbound warfarin from 1.02% to 1.34% (P-value < 0.05) and lowered warfarin plasma concentration from 741 to 533 ng/ml (P-value < 0.05). There was a correlation between the plasma concentration of diflunisal and the percentage unbound warfarin [25]. It was studied the interaction of diflunisal with warfarin on the binding of warfarin to human serum albumin. Diflunisal and warfarin concentration ranged from 300 to 1,000 µM and the concentration of human serum albumin was 3 µM. Warfarin and diflunisal bind to human serum albumin for 99% and 98%, respectively, and diflunisal displaced warfarin from the human serum albumin [26]. Forty-eight patients received diflunisal at the dose of 500 mg twice-daily and the intraocular pressure was reduced from 3.8+3.1 to 1.6+1.5 mm Hg (P-value < 0.0001) in patients who also received acetazolamide. Diflunisal potentiated the ocular hypotensive effect of acetazolamide by increasing its unbound plasma concentration [27]. It was studied the displacement of salicylic acid from the human plasma proteins by diflunisal. At a diflunisal concentration of 50 μg/ml diflunisal was bound to plasma protein for 99.83% and displaced salicylic acid from plasma proteins [28]. Both diflunisal and indomethacin are glucoronated and diflunisal inhibited the glucuronidation of indomethacin in human liver microsomes with an IC50 ranging from 100 to 231 µM. In human liver microsomes, the inhibition of indomethacin glucuronidation by diflunisal was more potent with an IC50 of 15.2 to 48.7 µM. When diflunisal was administered at the dose of 250 mg twice-daily the concentration of diflunisal in the intestine is higher than the IC50 values of indomethacin [29]. Indomethacin was administered at the daily dose of 100 mg to 8 healthy volunteers who also received 500 mg of diflunisal in the morning and 1,000 mg of diflunisal in the evening. High dose diflunisal (1,500 mg daily) decreased the renal clearance of indomethacin from 21.9 to 1.8 ml/min (92%) and reduced the renal excretion of both unchanged indomethacin (63%) and glucuronidated indomethacin (82%). Diflunisal reduced the total body clearance and the distribution volume of indomethacin by 47% and by 35%, respectively, and diflunisal increased the peak plasma concentration and the area under the concentration-time curve of indomethacin by 40% and by 119%, respectively. These results indicate that diflunisal interacts with indomethacin and the interaction occurs at both the metabolic and excretory levels [30]. Diflunisal was administered at the dose of 250 mg twice-daily for 15 days to 8 healthy volunteers and probenecid was co-administered at the dose of 500 mg twice-daily. The steady-state plasma concentration of diflunisal was 104 µg/ml when probenecid was administered and 63.1 µg/ml without probenecid (P-value < 0.05) and this increase was due to a significant decrease in the total body clearance of diflunisal from 5.8 to 3.4 ml/min. The formation clearances of diflunisal phenol glucuronide and diflunisal acyl glucuronide were decreased to 45% and to 54%, respectively, when probenecid was co-administered whereas the sulfation of diflunisal was not affected by probenecid. The steady-state plasma concentrations of diflunisal sulphate and diflunisal glucuronides were 2.5-fold higher and 3.1-fold higher, respectively, when probenecid was co-administration and this increase was due to a significant reduction in the renal clearance of the diflunisal conjugates [31].
Toxicity induced by diflunisal
Diflunisal is a safe drug and the information about the toxicity induced by diflunisal is poor. A 33-year-old man ingested 14 grams of diflunisal, 3 tablets of propoxyphene-acetaminophen, and ethanol. Toxicology screening revealed marked elevated plasma salicylate concentration of 1.76 mg/ml, 1.0 mg/ml, and 0.41 mg/ml at 4.5, 10, and 24 hours, respectively, post-ingestion, and the plasma concentration of ethanol was 4.5 mg/ml. There was no respiratory alkalosis and metabolic acidosis and the patient recovered fully [32]. Diflunisal was administered at the daily dose of 500 to 1,000 mg and aspirin was administered at the daily dose of 2,000 to 4,000 mg to patients with rheumatoid arthritis. Both diflunisal and aspirin induced adverse-effects but the adverse-effects were fewer in patients who received diflunisal [33]. One-hundred-fifteen patients with osteoarthritis received either diflunisal at the daily dose of 700 mg or aspirin at the daily dose of 2,460 mg. Diflunisal produced fewer general adverse-effects (P-value < 0.01) and fewer gastrointestinal adverse-effects (P-value < 0.01) than aspirin. Fewer patients who received diflunisal (P-value < 0.05) discontinued the therapy because of adverse-effects than patients who received aspirin. Thus, diflunisal is safer than aspirin [34]. Twelve patients with acquired amyloid neuropathy who underwent liver transplantation were treated with diflunisal at the daily dose of 500 mg. These patients developed neurological deterioration by 12 months of treatment and the treatment was also associated with a high incidence of adverse-effects [35]. Diflunisal was administered at the daily dose of 500 mg to 40 patients with hereditary transthyretin and diflunisal was well-tolerated but the renal function and the blood cell counts must be carefully monitored [36]. Diflunisal was administered at the daily dose of 500 mg to 25 patients with rheumatoid arthritis and diflunisal was generally well-tolerated but induced gastrointestinal effects which were mild. Adverse-effects have been reported in about 20% of patients in the first 12 weeks of treatment and in about 7 % of patients who received diflunisal for longer treatment. Central nervous system adverse-effects, such as dizziness and headache occurred in about 5% of patients whilst tinnitus rarely occurred [37].
Discussion
Diflunisal is a difluorophenyl derivative of salicylic acid, is not converted to salicylic acid, competitively inhibits cyclooxygenase, and is a potent anti-inflammatory drug but is devoid of antipyretic effects because of the poor penetration into the cerebrospinal fluid. Diflunisal has been used as an analgesic in treatment of osteoarthritis and musculoskeletal strains or sprains. In adults, the oral dose of diflunisal is 250 to 500 mg twice-daily or thrice-daily and the elimination half-life of diflunisal is about 10 hours. Diflunisal is rapidly absorbed following oral administration, reaches the peak concentration in about 2 to 3 hours, is bound to plasma protein for 99%, and is distributed throughout most body tissues and transcellular fluids primary by pH-dependent processes. Diflunisal is extensively metabolized as it is conjugated with sulfate and with glucuronic acid [1]. The efficacy and safely of diflunisal have been reviewed. Diflunisal, administered at the dose of 250 mg twice-daily, effectively and safely treats patients with neuropathy and cardiomyopathy [2], diflunisal, administered at the daily dose of 500 mg, effectively and safely treats patients with familial amyloid polyneuropathy [3], a single dose of 1,000 mg of diflunisal effectively and safely treats surgical pain in patients who underwent the removal of third molars [4], diflunisal effectively and safely treats postsurgical pain [5], and the oral ointment of 2% of diflunisal is more effective (P-value < 0.014) than 2% aspirin oral ointment in treatment of painful oral mucous lesions [6]. The treatment with diflunisal has been reviewed. Diflunisal, administered at the daily dose of 500 mg to patients with transthyretin amyloid cardiomyopathy, improves survival-rate and overall stability in clinical and echocardiographic markers of disease [7], diflunisal, administered at the daily dose of 500 mg, treats patients with transthyretin amyloidosis and patients with hereditary transthyretin amyloidosis [8], diflunisal, administered at the dose of 500 mg twice-daily, treats patients with amyloid disease who were allergic to ibuprofen [9], diflunisal, administered at the dose of 250 mg twice-daily, treats patients with transthyretin amyloidosis cardiomyopathy [10], three doses of 500 mg of diflunisal are more effective than placebo (P-value < 0.0001) in extinguishing pain in patients who underwent impacted mandibular third molar [11], diflunisal, administered at the daily dose of 500 to 750 mg, is more effective than ibuprofen, administered at the daily dose of 800 to 1,200 mg, in treatment of patients with osteoarthritis [12], diflunisal, administered at the dose of 500 mg twice-daily, is effective and well-tolerated as naproxen, administered at the dose of 375 mg twice-daily, in patients with active rheumatoid arthritis [13], and diflunisal, administered at the daily dose of 500 mg to patients with osteoarthritis, is better tolerated, causes fewer adverse-effects, and less increase in cell and lysosomal enzyme excretion by the kidney than aspirin administered at the daily dose of 500 mg [14]. The trials conducted with diflunisal have been reviewed. A single-arm, open-label trial was conducted with diflunisal, administered at the dose of 250 mg twice-daily, to patients with wild-type or mutant transthyretin cardiac amyloidosis and diflunisal is well-tolerated and treats the patients [15], a randomized, clinical trial was conducted in patients with familial amyloid polyneuropathy who received diflunisal at the dose of 250 mg twice-daily or placebo. Diflunisal is more effective than placebo (P-value < 0.05) in the progression of neurological impairment and preserves the quality of life [16], a randomized, placebo-controlled, clinical trial was conducted in patients with active familial amyloidotic polyneuropathy and diflunisal controls the neurologic disease progression and treats the patients [17], an international, randomized, placebo-controlled trial was conducted in patients with active familial amyloidotic and diflunisal is well-tolerated and treats the patients [18], a 12-week, double-blind, controlled trial compared the efficacy of diflunisal to that of naproxen in patients with rheumatoid arthritis. Both drugs were administered at the daily dose of 500 mg, improve rheumatoid arthritis, and diflunisal causes fewer adverse-effects than naproxen [19], and two double-blind, inter-group trials were conducted in patients with osteoarthritis of the knees and hips who received diflunisal, naproxen, or aspirin at the daily dose of 500 mg. Aspirin was administered for 12 weeks and diflunisal and naproxen were administered for 8 weeks. Diflunisal is better tolerated than naproxen and aspirin and treats the patients [20]. De Schepper et al. [21] studied the pharmacokinetics of diflunisal 5 healthy volunteers, in 9 patients with moderate renal insufficiency, in 3 patients with preterminal renal insufficiency, and in 5 patients with terminal renal insufficiency, and a single oral dose of diflunisal of 500 mg was administered to healthy volunteers and to patients. The elimination half-life of diflunisal is 10.8+8.0, 22.4+2.5, 59.6+3.3, and 115+13.9 hours in healthy volunteers, in patients with moderate renal insufficiency, in patients with preterminal renal insufficiency, and in patients with terminal renal insufficiency, respectively. These results indicate that the elimination half-life of diflunisal is shorter in healthy volunteers than in patients and increases with the degree of renal impairment. In addition, all pharmacokinetic parameters of diflunisal are impaired in patients and vary with the degree of renal impairment. Loewen et al. [22] studied the metabolism of diflunisal in 6 healthy volunteers who received a single oral dose of 100, 250, 500, 750, or 1,000 mg of diflunisal. Diflunisal is conjugate with sulfate and with glucuronic acid, the glucuronidation of diflunisal is predominant over the sulfation, and consists in diflunisal phenol glucuronide and diflunisal acyl glucuronide. Most of diflunisal and its metabolites are recovered in the urine and the urinary recovery of diflunisal sulfate increases with the dose, that of diflunisal phenolic glucuronide is unaffected by the dose, and that of acyl glucuronide decreases with the dose. Herman et al. [23] studied the effects of contraceptive consumption and cigarette smoking on the metabolism of diflunisal in subjects who received a single dose of 50 mg of diflunisal and the urinary excretion of diflunisal sulfate, diflunisal acyl glucuronide, and diflunisal phenolic glucuronide was assessed. Females using oestrogen-containing oral contraceptives excreted 50% less diflunisal sulfate and 20% more diflunisal acyl glucuronide than non-users and the recovery of diflunisal sulfate was reduced by about 30% by cigarette smokers. These results indicate that the consumption of contraceptives and cigarette smoking affect the metabolism of diflunisal. Macdonald et al. [24] studied the effects of liver cirrhosis on the pharmacokinetics and metabolism of diflunisal. Five patients with liver cirrhosis and in 5 healthy volunteers received a single dose of 250 mg of diflunisal. The unbound fraction of diflunisal is increased in cirrhotic patients, the total body clearance of unbound diflunisal is reduced in cirrhotic patients, the unbound partial clearances of phenol and acyl glucuronides are decreased in cirrhotic patients by approximately 38%. These results indicate that the metabolism and the pharmacokinetic parameters of diflunisal are impaired in patients with liver cirrhosis. The interaction of diflunisal with drugs has been reviewed. Diflunisal, administered at the dose of 500 mg twice-daily for 2 weeks, increases the unbound fraction of warfarin (P-value < 0.05), lowers the plasma concentration of warfarin (P-value < 0.05), and there is a correlation between the plasma concentration of diflunisal and the unbound fraction of warfarin [25], diflunisal displaces warfarin from the human serum albumin [26], diflunisal, administered at the daily dose of 500 mg twice-daily, potentiates the ocular hypotensive effect of acetazolamide (P-value < 0.0001) by increasing the unbound plasma of acetazolamide [27], diflunisal displaces salicylic acid from the human plasma protein [28], diflunisal and indomethacin are glucuronidated and diflunisal inhibits the glucuronidation of indomethacin in human liver microsomes. When diflunisal was administered at the dose of 250 mg twice-daily the concentration of diflunisal in the intestine is higher than the IC50 values of indomethacin [29], indomethacin was administered at the daily dose of 100 mg and diflunisal was co-administered at the dose of 500 mg in the morning and at a dose of 1,000 mg in the evening to healthy volunteers. Diflunisal decreases the renal clearance of indomethacin, the renal excretion of both unchanged and glucoronated indomethacin, the total body clearance and the distribution volume of indomethacin and diflunisal increases the peak plasma concentration and the area under the concentration-time of indomethacin [30], and diflunisal was administered at the dose of 250 mg twice-daily for 15 days to healthy volunteers and probenecid was co-administered at the dose of 500 mg twice-daily. Probenecid increases the steady-state plasma concentration of diflunisal and this increase is caused by the reduction of the total body clearance of diflunisal. The formation clearances of diflunisal phenol glucuronide and diflunisal acyl glucuronide are decreased by probenecid whereas the sulfation of diflunisal is not affected by probenecid. The steady-state concentrations of diflunisal sulfate, diflunisal phenol glucuronide and diflunisal acyl glucuronide are increased by probenecid [31]. The toxicity induced by diflunisal has been reviewed. Diflunisal is a safe drug and the information about the toxicity induced by diflunisal is poor. A man ingested 14 grams of diflunisal, 3 tablets of propoxyphene-acetaminophen, and ethanol. The highest plasma concentration of salicylate was 1.76 mg/ml and that of ethanol was 4.5 mg/ml. There was not respiratory alkalosis and metabolic acidosis and the patient recovered fully [32], diflunisal was administered at the at the daily dose of 500 to 1,000 mg and aspirin was administered at the daily dose of 2,000 to 4,000 mg to patients with rheumatoid arthritis and diflunisal induced fewer adverse-effects than aspirin [33], diflunisal was administered at the daily dose of 700 mg and aspirin was administered at the daily dose of 2,460 mg. Diflunisal induced fewer adverse-effects (P-value < 0.01) and fewer gastrointestinal adverse-effects (P-value < 0.001) than aspirin and fewer patients who received diflunisal (P-value < 0.05) discontinued therapy because of adverse-effects than patients who received aspirin thus diflunisal is safer than aspirin [34], patients with acquired amyloid neuropathy who underwent liver transplantation received diflunisal at the daily dose of 500 mg. By 12 months of treatment patients developed high incidence of adverse-effects [35], diflunisal was administered at the daily dose of 500 mg to patients with hereditary transthyretin and diflunisal was well-tolerated but the renal function and the blood cell counts must be carefully monitored [36], and diflunisal was administered at the daily dose of 500 mg to patients with rheumatoid arthritis and diflunisal was well-tolerated but induced gastrointestinal adverse-effects which were mostly mild. Adverse-effects have been reported in about 20% of patients in the first 12 weeks of treatment and in about 7% of patients who received diflunisal for longer treatment. Central nervous systemic adverse-effects such as dizziness and headache occurred in 5% of patients while tinnitus rarely occurred [37].
In conclusion, diflunisal is a difluorophenyl derivative of salicylic acid, is not converted to salicylic acid, competitively inhibits cyclooxygenase, and is a potent anti-inflammatory drug. Diflunisal has been used as an analgesic in treatment of osteoarthritis and musculoskeletal strains or sprains. In adults, the oral dose of diflunisal is 250 to 500 mg twice-daily or thrice-daily, the elimination half-life of diflunisal is about 10 hours, and diflunisal is rapidly absorbed. The efficacy and safely of diflunisal, the treatment with diflunisal, and the trials conducted with diflunisal have been reviewed. In addition, the metabolism of diflunisal, the pharmacokinetics of diflunisal, the interaction of diflunisal with drugs, and the toxicity induced by diflunisal have been reviewed. The pharmacokinetics of diflunisal have been studied in healthy volunteers and in patients with renal insufficiency and the pharmacokinetic parameters are impaired in patients. The aim of this study is to review the clinical pharmacology of diflunisal.
Conflict of interests: The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria.
This article is a review and drugs have not been administered to men or animals.
Acknowledgments: The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
References
- Grosser T, Ricciotti E, FitzGerald GA. Pharmacotherapy of inflammation, Fever, Pain, and Gout, In Goodman@Gilman’s. The Pharmacological Basis of Therapeutics. Brunton LL, Knollmann BC editors. Mc Graw Hill, 14th 25023: pp 829-856.
- Obici L, Cortese A, Perlini S, Lozza A, Casarini S, Alfonsi E, et al. Diflunisal in late-onset FAP patients with moderate to severe neuropathy. Orphanet J Rare Dis, 2015; 10(Suppl 1): 24-28.
- Sekijima Y, Tojo K, Morita H, Koyama J, Koike H, Sobue G, et al. Safety and Efficacy of Long-Term Diflunisal Administration in Familial Amyloid Polyneuropathy (PD1.009). Neurology, 2013; 80(7 suppl 1): https://doi.org/10.1212.
- Sisk AL, Mosley RO, Martin RP. Comparison of preoperative and postoperative diflunisal for suppression of postoperative pain. J Oral Maxillofacial Surg, 1989; 47(5): 464-468.
- Van Winzum C, Rodda B. Diflunisal: Efficacy in postoperative pain. Br J Clin Pharmacol, 1977; 4(2): 39S-43S.
- Kizu J, Tsuchiya M, Watanabe S, Yasuno N, Arakawa Y, H Saijyo H, et al. Preparation and clinical application of 2% diflunisal oral ointment for painful lesions of the oral mucosa. Yakugaku Zasshi, 2001; 121(11): 829-835.
- Siddiqi OK, Mints YY, Berk JL, Connors L, Doros G, Gopal DM, et al. Diflunisal treatment is associated with improved survival for patients with early stage wild-type transthyretin (ATTR) amyloid cardiomyopathy: the Boston University Amyloidosis Center experience. Amyloid, 2022; 29(2): 71-78.
- Ikram A, Donnelly JP, Sperry BW, Samaras C, Valent J, Icon V, et al. Diflunisal tolerability in transthyretin cardiac amyloidosis: a single center’s experience. J Protein Fold Dis, 2018; 2(6): 197-202.
- Aw J, Hui LS, Poh LC, Adrian CK. Successful Diflunisal Desensitization in a Transthyretin Amyloidosis Patient With Ibuprofen Allergy: A Case Report. Pharm Biomed Res, 2022; 8(3): 233-236.
- Lohrmann G, Pipilas A, Mussinelli R, Gopal DM, Berk JL, Connors LH, et al. Stabilization of Cardiac Function With Diflunisal in Transthyretin (ATTR) Cardiac Amyloidosis. J Card Fail, 2020; 26(9): 753-759.
- Petersen JK. Diflunisal, a new analgesic, in the treatment of postoperative pain following removal of impacted mandibular third molars. Int J Oral Surg, 1979; 8(2): 102-113.
- Umbenhauer ER. Diflunisal in the treatment of the pain of osteoarthritis. Summary of clinical studies. Pharmacotherapy, 1983; 3(2 Pt 2): 55S-60S.
- Osborn TG, Parks RL, Moore TL, Grisanti MW, Nesher G, Caciolo BA. Diflunisal versus naproxen in the management of rheumatoid arthritis. Clin Ther, 1989; 11(6): 736-743.
- Dieppe PA, Huskisson EC. Diflunisal and aspirin: a comparison of efficacy and nephrotoxicity in osteoarthritis. Rheumatol Rehabil, 1979; 18(1): 53-56.
- Castaño A, Helmke, Alvarez J, Delisle S, Maurer MS. Diflunisal for ATTR Cardiac Amyloidosis. Congest Heart Fail, 2012; 18(6): 315-319.
- Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA, 2013; 310(24): 2658-2667.
- Berk JL, Suhr OB, Sekijima Y, Yamashita T, Heneghan M, Zeldenrust SR, et al. The Diflunisal Trial: study accrual and drug tolerance. Amyloid, 2012; 19(Suppl 1): 37-38.
- Berk JL, Dyck PJ, Obici L, Zeldenrust SR, Sekijima Y, Yamashita T, et al. The diflunisal trial: update on study drug tolerance and disease progression. Amyloid, 2011; 18(Suppl 1): 196-197.
- Schorn D. Diflunisal in rheumatoid arthritis. A controlled trial. S Afr Med J, 1981; 60(24): 936-937.
- Grayson MF. Two trials of diflunisal in osteoarthritis. Curr Med Res Opin, 1978; 5(7): 567-571.
- De Schepper PJ, Mullie A, Tjandramaga TB, Verbeek R, Verberckmoes R, Grahame-Smith DG. Pharmacokinetics of diflunisal elimination in patients with renal insufficiency. Proceedings of the B.P.S, 1977; Page 644.
- Loewen GR, Herman RJ, Ross SG, Verbeeck RK. Effect of dose on the glucuronidation and sulphation kinetics of diflunisal in man: single dose studies. Br J Clin Pharmacol, 1988; 26(1): 31-39.
- Herman RJ, Loewen GR, Antosh DM, Taillon MR, Hussein S, Verbeeck RK. Analysis of polymorphic variation in drug metabolism: III. Glucuronidation and sulfation of diflunisal in man. Clin Inv Med, 1994; 17(4): 297-307.
- Macdonald JI, Wallace SM, Mahachai V, Verbeeck RK. Both phenolic and acyl glucuronidation pathways of diflunisal are impaired in liver cirrhosis. Eur J Clin Pharmacol, 1992; 42(5): 471-474.
- Serlin MJ, Mossman S, Sibeon RG, Tempero KF, Breckenridge AM. Interaction between diflunisal and warfarin. Clin Pharmacol Ther, 1980; 28(10): 493-498.
- Amézqueta S, Bolioli AM, Beltrán JL, Ràfols C. Evaluation of the interactions between human serum albumin (HSA) and warfarin or diflunisal by using molecular fluorescence using two approaches. Admet & Dmpk, 2018; 6(1): 47-54.
- Yablonski ME, Maren TH, Hayashi M, Naveh N, Potash SD, Pessah N. Enhancement of the ocular hypotensive effect of acetazolamide by diflunisal. Am J Ophthalmol, 1988; 106(3): 332-336.
- Verbeeck RK, Boel A, Buntinx A, De Schepper PJ. Plasma protein binding and interaction studies with diflunisal, a new salicylate analgesic. Biochem Pharmacol, 1980; 29(4): 571-576.
- Mano Y, Usui T, Kamimura H. In vitro drug interaction between diflunisal and indomethacin via glucuronidation in humans. Biopharm Drug Dispos, 2006; 27(6): 267-273.
- Eriksson LO, Wåhlin-Boll E, Liedholm H, Seideman P, Melander A. Influence of chronic diflunisal treatment on the plasma levels, metabolism and excretion of indomethacin. Eur J Clin Pharmacol, 1989; 37(1): 7-15.
- Macdonald JI, Wallace SM, Herman RJ, Verbeeck RK. Effect of probenecid on the formation and elimination kinetics of the sulphate and glucuronide conjugates of diflunisal. Eur J Clin Pharmacol, 1995; 47(6): 519-523.
- Duffens KR, Smilkstein MJ, Bessen HA, Rumack BH. Falsely elevated salicylate levels due to diflunisal overdose. J Emerg Med, 1987; 5(6): 499-503.
- de Silva M, Hazleman BL, Dippy JE. Diflunisal and aspirin: a comparative study in rheumatoid arthritis. Rheumatol Rehabil, 1980; 19(2): 126-130.
- Andrew A, Rodda B, Verhaest L, Van Winzum C. Diflunisal: six-month experience in osteoarthritis. Br J Clin Pharmacol, 1977; 4 (Suppl 1): 45S-52S.
- Nedkova-Hristova V, Baliellas C, González-Costello J, Lladó L, González-Vilatarsana E, Vélez-Santamaría V, et al. Treatment With Diflunisal in Domino Liver Transplant Recipients With Acquired Amyloid Neuropathy. Transpl Int, 2022; 35: 10454. doi: 10.3389.
- Sekijima Y, Tojo K, Morita H, Morita H, Ikeda S-I. Safety and efficacy of long-term diflunisal administration in hereditary transthyretin (ATTR) amyloidosis. J Prot Folding Disor, 2015; 22(2): 79-83.
- Brogden RN, R Heel RC, Pakes GE, Speight TM, Avery GS. Diflunisal: a review of its pharmacological properties and therapeutic use in pain and musculoskeletal strains and sprains and pain in osteoarthritis. Drugs, 1980; 19(2): 84-106.

