A Comparative Study Between LMA BlockBuster™ and LMA ProSeal™ in Patients Undergoing Operative Procedures Under General Anaesthesia – A Prospective Randomised Controlled Study
Ayushi Saxena* and Syed Moied Ahmed
Department of Anaesthesiology and Critical Care, JNMCH, AMU, India
Received Date: 25/07/2024; Published Date: 16/10/2024
*Corresponding author: Ayushi Saxena, Department of Anaesthesiology and Critical Care, JJawaharlal Nehru Medical College Hospital, Aligarh Muslim University, India
Abstract
Background and Aims: LMA ProSeal™, a second generation Supraglottic Airway Device (SAD), has been established as ‘gold standard’ by many studies due to its easy insertion, ability to sustain higher airway seal pressures and lesser complications. To ascertain the clinical efficacy of LMA BlockBuster™(BLMA) as compared to LMA ProSeal™(PLMA), in terms of time taken for insertion, number of attempts, position placement with fibreoptic laryngoscope, Oropharyngeal Leak Pressure (OLP), haemodynamic changes and post-insertion complications, we conducted this study.
Methods: Ninety ASA Grade I & II patients of either sex, 20-70years old, weighing between 50-70kg and of all MP Classes, scheduled for various elective surgical procedures under general anaesthesia were included in the study and were randomly divided in the BLMA and PLMA group. After uniformly administering anaesthesia, respective SAD was inserted and various parameters were recorded and compared.
Results: The time required to insert BLMA was found to be significantly less than PLMA(p<0.001). The OLP was found to be significantly higher in BLMA as compared to PLMA(p<0.001). There was no significant difference in ease of insertion, Brimacombe score and post operative complications. There was a significant change in heart rate and mean arterial blood pressure after insertion of BLMA. However, the difference between the two devices was not significant.
Conclusion: Our study suggests that LMA BlockBuster™ has clinical efficacy superior to that of LMA ProSeal™ in terms of time taken for insertion and OLP; and similar to it in terms of glottic visualisation, haemodynamic changes and post-insertion complications.
Keywords: Clinical efficacy, fibreoptic laryngoscope, haemodynamic, general anaesthesia
Introduction
The limitations of the first generation supraglottic airway devices (SADs), paved way for the development of second generation supraglottic airway devices [1,2]. Due to its easy insertion, ability to sustain higher airway seal pressures, a gastric drain tube that helps in Ryle’s tube insertion and lesser complications, LMA ProSealTM (PLMA) [3] has been established as a ‘gold standard’ supraglottic airway device by many studies.
However, in the recent years many newer second-generation devices have been introduced [2]. LMA BlockBuster™ (Figure 1) is one such modification which has a preformed shape and has been proved to be clinically superior to other supraglottic airway devices by various studies. However, no study in the published literature was found where the BLMA has been directly compared with PLMA. It is, thus, hypothesised that BLMA could prove to be a clinical more efficacious device than PLMA due to its preformed shape enabling faster insertion. Also, PLMA has to be inserted using an introducer which on removal may lead to slight displacement of the device causing leakage of ventilatory gases and improper position over glottic opening.
Hence, this study was conducted to compare clinical efficacy of LMA BlockBuster™ with that of LMA ProSealTM in terms of time taken for insertion as primary objective and number of attempts, position placement with fibreoptic laryngoscope, oropharyngeal leak pressure, haemodynamic changes and post-insertion complications as secondary objectives.

Figure 1: LMA BlockBusterTM.
Method
After approval from the Board of studies and JNMCH institutional ethical committee (D.No 349 on 19.06.2021), this prospective randomized study was conducted at a tertiary care hospital over a period of 2 years according to the principles of the Declaration of Helsinki, 2013. The study was registered at Clinical Trial Registry of India (CTRI/2022/10/04634, on 11/10/2022). Based on previous studies [4,11], we choose time of insertion as the primary criteria for calculating our sample size at 80% power and 95% confidence level; which came as 42 in each group to detect a mean difference of 15%. We recruited 90 patients for the study to overcome the dropouts as shown in consort chart (Figure 2) after obtaining written informed consent for participation in the study and use of the patient data for research and educational purposes.
Ninety patients of ASA grade I and II patients of either sex, aged 20-70years, weighing 40-70kg, of all MP (Mallampatti) grades, undergoing operative procedures under general anaesthesia were included in the study. Patients who had any pathology of the oral cavity that may obstruct the insertion of device, mouth opening less than 2.5cm, potentially full stomach patients (trauma, morbid obesity, history of gastric regurgitation and heart burn, full term pregnancy), patient with disrupted upper airway, facial or upper airway trauma, burns following caustic ingestion, patients with stiff lungs, those at risk of oesophageal reflux (hiatus hernia), had bleeding disorder or those who were unable to provide informed consent, were not included in the study.
Patients were randomly divided into 2 groups using simple randomisation by chit in a box method [5] (each of which had either of the two letters, A or B, written and one of these was opened by the assistant after induction of anaesthesia):
- Group A (PLMA or Control group) [n =45] underwent LMA ProSeal™ insertion.
- Group B (BLMA or Study group) [n =45] underwent LMA BlockBuster™ insertion.
The study was single-blinded as the patient was unaware of the group being assigned to him and allocation concealment was done using Sequentially Numbered, Opaque, Sealed Envelopes (SNOSE).
Insertion learning curve was achieved by performing 15 insertions on manikin and 10 insertions on patients, using each of the devices prior to start of study.
A detailed Pre-Anaesthetic Check-up (PAC) including history, clinical examination and routine investigations were carried out in all patients to ensure that they met the inclusion criteria and were eligible for our study. The demographic data such as age, weight, height, BMI (Body Mass Index), Mallampati Grading (MP Grade), etc were noted.
Patients were kept nil per oral (NPO) for 8 hours prior to surgery. After arrival in the OT, an 18G/20G peripheral intravenous catheter was secured and standard multichannel monitor was connected. Uniform premedication was done as per our institutional protocol, with Inj. Midazolam 0.03 mg/kg, Inj Dexamethasone 0.1 mg/kg and Inj Fentanyl 2.0 mcg/kg of body weight. After pre-oxygenation with 100% oxygen for 3 min, the patient was induced with Inj. Propofol 2.0 mg/kg and titrated to achieve an end point. After adequate muscle relaxation with Inj. Succinylcholine 1.5 mg/kg, device insertion was carried out depending on the group to which the patient was assigned. The appropriate size of SAD was selected according to body weight as per the manufacturers' guidelines. Soon after the insertion, the cuff of SAD was inflated with air and connected to the breathing circuit. Successful placement of SAD was confirmed by the ability to achieve tidal volume of at least 7-8 ml/kg with a square wave Capnogram. Adequate ventilation was defined by easy bag ventilation, bilateral equal air entry, absence of audible air leak around the cuff, adequate chest rises, airway pressure of Peak pressure<30mmH2O, Oxygen saturation>90% and end tidal CO2 of 35-45 mmHg. Outcome measures were noted.
The primary outcome measure was the time taken for insertion which was defined from the time when the device crossed the incisors in first attempt till adequate ventilation was established as confirmed by adequate chest expansion and normal square wave capnogram [4].
All the attempts in which the supraglottic airway device crossed the incisor was considered as an attempt and recorded as secondary outcome measure. A maximum of two attempts were taken after which if adequate ventilation with chest expansion and a square wave form capnogram could not be achieved, it was declared as ‘failure to device insertion’, but the patient was included in the study and some other device or technique was used to secure the airway of that patient.
The ease of insertion was assessed using a subjective scale of 1-3 [6] (Grade: Easy - Insertion within pharynx in single attempt with no resistance, Grade 2: Difficult - Resistance to insertion felt or more than 1 attempt required, Grade 3: Impossible - Inability to place the device)
Airway sealing pressure or Oropharyngeal Leak Pressure (OLP) was measured for both the devices by closing the expiratory valve of the circle system at a fixed gas flow rate of 3 L/min (to a maximum of 40cmH2O). A gradual rise of airway pressure shown in the ventilator monitor with a sudden fall which corresponded with the audible noise lateral to the thyroid cartilage was recorded as OLP [3,7].
The position placement of the device was graded by Brimacombe score [8], that has been introduced for grading of glottic view which signifies the ease of intubation through the SAD, using fibreoptic scope through the airway tube distal to the mask opening (Figure 3). The view is scored as follows: 4-only cords seen; 3-cords plus posterior epiglottis seen; 2-cords plus anterior epiglottis seen; 1-cords not seen, but function adequate; 0-failure to function where cords not seen through the fibreoptic scope.
Haemodynamic changes (pulse rate, MABP- Mean Arterial Blood Pressure, SpO2) were noted before and after (immediately, 1min & 5min) device insertion. Surgery was allowed to commence only after the collection of the last haemodynamic data at 5 minutes post-insertion. After the surgery, the residual neuromuscular paralysis was reversed and postoperative complications were noted. Blood staining on the device was recorded as presence or absence of blood on the supraglottic airway device. Sore throat was analysed after removal of the device and defined as pain, scratchiness or irritation of throat assessed by 4-point scale: (1: No sore throat, 2: mild- complains of sore throat only on inquiry, 3: moderate- complaints of sore throat without inquiry, 4: severe- sore throat with soreness and associated with throat pain). Post-op Nausea Vomiting was assessed presence or absence of forceful expulsion of contents of the stomach out through the mouth. All the parameters were recorded by a single observer all throughout the study, who was not a part of the study.
Categorical variables (sex, MP Grading, type of surgery, number of attempts, ease of insertion, Brimacombe score, etc) are presented in the form of number and percentage (%). Quantitative data (age, weight, height, BMI, time of insertion, OLP, etc.) are presented as the means (SD and median with 25th and 75th percentiles (interquartile range). Normality of data was checked by using Kolmogorov-Smirnov test. The cases in which the data was not normal, non-parametric tests were used. Non parametric categorical data like gender, number of attempts, post operative complications were analysed using the Chi square test. Non parametric ordinal categorical data like mallampati grade, ease of intubation was analysed by Mann-Whitney test. Parametric data like age, weight, time taken and haemodynamic changes were analysed using the Paired and Unpaired t-test as per data. Data entry was done in MS Excel spreadsheet 365 version 2211. All the tests were performed using computer program SPSS version 25.0. A p < 0.05 at 95% confidence interval was considered statistically significant.
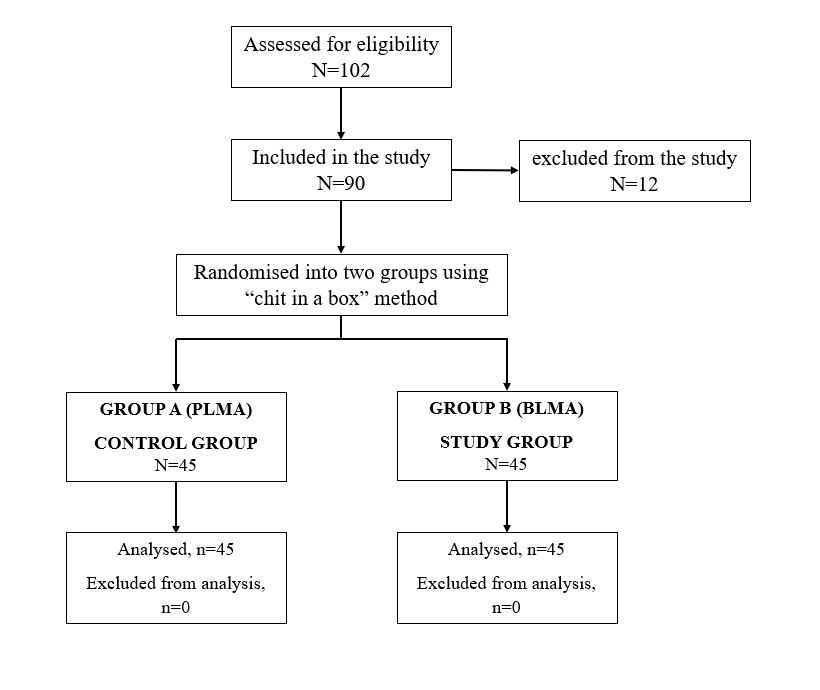
Figure 2: Consort Chart.
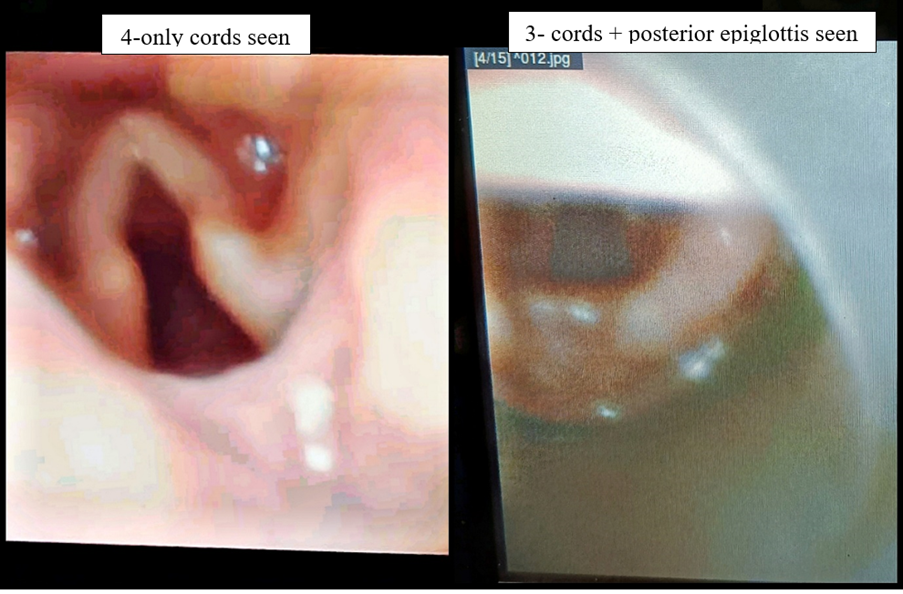
Figure 3: Brimacombe scoring by fibreoptic scope.
Results
A total of 90 patients who were included for participation in the study had comparable demographic characteristics and underwent surgeries of comparable nature (Table 1).
In Group A (PLMA) patients the LMA ProSeal™ was secured in 20.97(4.40) sec, while in group B (BLMA) patients, the time required was 14.37(4.40) sec (Table 2). This difference was statistically significant (p<0.001). The PLMA was cured in first attempt in 71.1% cases and failure of device insertion occurred in 8.9% patients. While in group B (BLMA), 77.8% of patients had their airways secured in the first attempt and 6% had failure of device insertion (Table 2). However, this difference was not found to be significant. There was no significant difference found in the ease of insertion (Table 2) and Brimacombe score (Table 2, Figure 3) between both the groups. The oropharyngeal leak pressure was significantly higher (p<0.001) in group B (BLMA), ie. 38.00(3.21) mmHg, as compared to group A (PLMA), ie. 32.59(3.2) mmHg (Table 2). There was a significant change in heart rate and mean arterial blood pressure with LMA BlockBuster™, however, the difference in terms of haemodynamic changes in between the two groups was not found to be statistically different (Table 2). Post operative complications were also not significantly different with LMA ProSeal™ and LMA BlockBuster™ (Table 2).
Table 1: Comparison of demographic characteristics.

Values are presented as mean(x±SD), percentage or median (1Q, 3Q)
1PLMA- LMA Proseal,
2BLMA- LMA BlockBuster
3MP Class- Mallampatti Class
4Lap chole- Laproscopic cholecystectomy
5Lap hernia repair- Laproscopic hernia repair
6MRM- Modified radical mastectomy
7N/O Humerus- Neck of humerus
Table 2: Comparision of outcome measures.
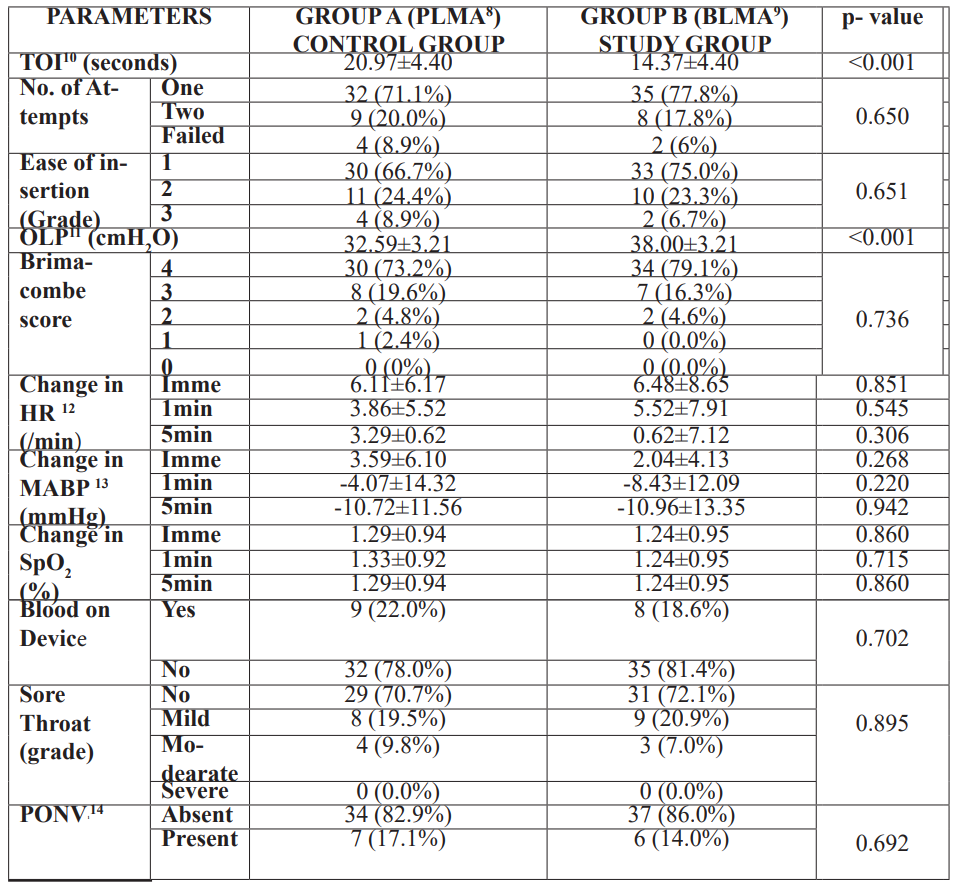
Values are presented as mean(x±SD), percentage or median (1Q, 3Q)
8PLMA- LMA Proseal
9BLMA- LMA BlockBuster
10 TOI- Time of insertion
11 OLP- Oropharyngeal leak pressure
12HR – heart rate
13 MABP- mean arterial blood pressure
14 PONV – Post operative nausea vomiting
Discussion
As suggested by our prior hypothesis, the primary outcome that is the time required to insert BLMA was found to be significantly less than PLMA. The OLP was found to be significantly higher in BLMA as compared to PLMA. There was no significant difference in ease of insertion, Brimacombe score and post operative complications. There was a significant change in heart rate and mean arterial blood pressure after insertion of BLMA. However, the difference between the two devices was not significant.
The patients were enrolled with similar demographics, in accordance to the previous studies we referred to (Table 1). Thus, enabling us to compare the results of our study with that of the previous studies, as the effect of demographic data on results was nullified.
Since LMA Block buster is a relatively new device so there was no study in the published literature where the BLMA has been directly compared with PLMA. Therefore, we analysed our findings with other studies where the BLMA [4,7,18] and PLMA [9-14] have been separately compared with other SAD and the superiority has been assessed between the two.
In our study, the time required for insertion was found to be 14.37(4.40) sec in BLMA group as compared to 20.97(4.40) sec in PLMA (p<0.001, Table 2). The insertion time of BLMA in our study was within the range of the previous studies which ranged from 12.2(1.5) sec to 15.92(3.02) sec [4,7,15]. However, in one study [12] the insertion time was higher than our study. This could be probably due to method of calculation of time of insertion. They considered the time of insertion from the time of picking up the device till confirmation of ventilation with capnogram waves. In other studies, the results for PLMA were similar to our study.
The oropharyngeal leak pressure with BLMA was significantly higher than PLMA in this study (Table 2, p<0.001). This was in accordance with the previous studies where both the devices BLMA and PLMA, has been compared separately with other SAD. However, the OLP with BLMA in the previous studies ranged between 26.65(1.59) cmH2O to 30.82(3.96) cmH2O which was lower than that in our study.
In this study, subjective grading system was used to assess the ease of insertion [6]. The overall success rate and incidence of first attempt insertion with BLMA was higher than with PLMA. However, it did not achieve statistical significance (Table 2). This was again in accordance with the studies where BLMA [4,7,15] and PLMA [9-14] has been compared separately with other supraglottic devices.
Various scoring systems have been introduced for grading of glottic view which signifies the ease of intubation through the SAD, such as Brimacombe score [8], POGO score [16], VCI score [17], etc. In this study we have referred to the Brimacombe score [8] as it is the most standardized. The Brimacombe score with BLMA was found to be higher as compared to PLMA but it did not achieve statistical significance (Table 2). This was similar to the previous studies where Brimacombe score of BLMA and PLMA was assessed against other SADs [4,7-13].
The haemodynamic changes and postoperative complications were found to be comparable between BLMA and PLMA (Table 2). This was in accordance with the previous studies [4,7-13].
No study is free from limitations. Similarly, our study had various limitations. Only patients undergoing elective surgeries and short duration, of average built and without any respiratory co-morbidity were included in the study. Thus, the clinical performance of the SADs in emergency surgeries with anticipated full stomach, long duration surgeries, obese patients, and patients with respiratory co-morbidity could not be commented upon. In addition, all the surgeries were performed in supine position. Thus, the effect of position on the study parameters could not be noted.
Every study has an element of bias. Our study was not an exceptional. It was a single-blinded study and was, thus, prone to have observer bias especially in subjective parameters such as time of insertion, the ease of insertion and the success rate. We, maintained a single observer all through the study, who was also not a part of the study to overcome the bias and observer variability. Other parameters OLP and haemodynamic changes were recorded through the electronic monitor.
Conclusion
Based on the findings of our study, we conclude that LMA BlockBuster™ is a better supraglottic device as compared to LMA ProSeal™. Further, we suggest that LMA BlockBuster™ will be a good option in situations when rapid insertion with good success rate in first attempt is required and when mechanical ventilation requires high airway pressures.
Acknowledgments:
Assistance with the study:
Financial support and sponsorship: None
Conflicts of interest: There are no conflicts of interest
Presentation: 12th National Airway Conference AIDAA (2021)
Contributions of authors:
Prof Syed Moied Ahmed: Concepts, Design, The definition of intellectual content, Manuscript editing
Ayushi Saxena: Literature search, Clinical studies, Experimental studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation
References
- Cook TM, Editorial I. The classic laryngeal mask airway: a tried and tested airway. What now? British Journal of Anaesthesia, 2006; 96(2): 149-152.
- Hernandez MR, Klock Jr PA, Ovassapian A. Evolution of the extraglottic airway: a review of its history, applications, and practical tips for success. Anesthesia & Analgesia, 2012; 114(2): 349-368.
- Brain AI, Verghese C, Strube PJ. The LMA ‘ProSeal’—a laryngeal mask with an oesophageal vent. British Journal of Anaesthesia, 2000; 84(5): 650-654.
- Das PP. Comparison of clinical performance of I-GEL and LMA blockbuster in adult patient during general anaesthesia: a comparative randomised trial. Indian Journal of Anaesthesia, 2022; 66.
- Verma S, Sharma SP. Effectiveness of Proseal laryngeal mask airway and laryngeal tube suction in elective non-laparoscopic surgeries of up to ninety minutes duration: A prospective, randomized study. Journal of Anaesthesiology Clinical Pharmacology, 2018; 34(1): 58-61.
- Radhika KS, Sripriya R, Ravishankar M, Kumar VH, Jaya V, Parthasarathy S. Assessment of suitability of i-gel and laryngeal mask airway-supreme for controlled ventilation in anesthetized paralyzed patients: A prospective randomized trial. Anesthesia Essays and Researches, 2016; 10(1): 88-93.
- Gao X, Liu JH, Chen CM, Wang Y, Wang ZY, Yan CL, et al Comparison of the supraglottic airway device BlockBusterTM and laryngeal mask airway supreme in anaesthetised, paralyzed adult patients: A multicenter randomized controlled trial. Expert Review of Medical Devices, 2022; 19(8): 649-656.
- Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesthesia & Analgesia, 1993; 76(2): 457.
- Tan Y, Jiang J, Wang R. Contrast of oropharyngeal leak pressure and clinical performance of I-gel™ and LMA ProSeal™ in patients: A meta-analysis. PLoS One, 2022; 17(12): e0278871.
- Singh A, Kaur J, Kaur S, Gupta KK. Safety and efficacy of LMA Supreme™ vs. LMA ProSeal™ for ambulatory surgeries in adult patients. Anaesthesia, Pain & Intensive Care, 2022; 26(1): 63-68.
- Singh I, Gupta M, Tandon M. Comparison of clinical performance of I-Gel™ with LMA—Proseal™ in elective surgeries. Indian journal of anaesthesia, 2009; 53(3): 302-305.
- Luthra A, Chauhan R, Jain A, Bhukal I, Mahajan S, Bala I. Comparison of two supraglottic airway devices: i-gel airway and proseal laryngeal mask airway following digital insertion in nonparalyzed anesthetized patients. Anesthesia Essays and Researches, 2019; 13(4): 669-675.
- Shin HW, Yoo HN, Bae GE, Chang JC, Park MK, You HS, et al. Comparison of oropharyngeal leak pressure and clinical performance of LMA ProSeal™ and i-gel® in adults: meta-analysis and systematic review. Journal of International Medical Research, 2016; 44(3): 405-418.
- Verma S, Sharma SP. Effectiveness of Proseal laryngeal mask airway and laryngeal tube suction in elective non-laparoscopic surgeries of up to ninety minutes duration: A prospective, randomized study. Journal of Anaesthesiology Clinical Pharmacology, 2018; 34(1): 58-61.
- Khare A, Awana P, Thada B, Mathur V, Kumar P. A Randomized comparative study to observe the safety and efficacy of I gel and blockbuster laryngeal mask airway used in patients undergoing short surgical procedure under general anesthesia. InThe Indian Anaesthetists Forum, 2022; 23(2): pp. 111-117.
- Park S, Lee HG, Choi JI, Lee S, Jang EA, Bae HB, et al. Comparison of vocal cord view between neutral and sniffing position during orotracheal intubation using fiberoptic bronchoscope: a prospective, randomized cross over study. BMC anesthesiology, 2019; 19: 1-7.
- Chaggar RS, Shah SV, Berry M, Saini R, Soni S, Vaughan D. The Video Classification of Intubation (VCI) score: a new description tool for tracheal intubation using videolaryngoscopy: a pilot study. European Journal of Anaesthesiology| EJA, 2021; 38(3): 324-326.

