The Art of Cranioplasty: Implants
Andia Shahzadi*
Jamaica Hospital Medical Center, NY
Received Date: 19/07/2024; Published Date: 15/10/2024
*Corresponding author: Andia Shahzadi, Jamaica Hospital Medical Center, NY
Abstract
Cranioplasty, the replacement of the skull defect following a craniectomy, has been around for thousands of years. Over centuries, the evolution of brain implants has led to outcomes that would give the patients second chance in life not only by preventing the shortening of their life span, but giving them the same if not better life quality as before the procedure. This paper reviewed the evolution of cranial implants and discuss the best options currently available depending on the patient's need.
Introduction
Neurosurgical patients that undergo craniectomy for the treatment of traumatic brain injury, cerebrovascular disease, or tumors are often left with large cranial defects post-operation. Such defects are required to allow the brain swelling to be reduced before the defect can be corrected. The technique by which it is reconstructed, referred to as cranioplasty, can have a lifelong impact on a patient's life. Exposed soft tissue, which includes the dura and brain, post-craniectomy is associated with risks such as traumatic injury to the unprotected area, increased likelihood of pseudomeningocele formation, and disrupted cerebrospinal fluid (CSF) flow dynamics. In addition to functional risks, cranial defects commonly disrupt the craniofacial architecture in ways that may lead to abnormal appearance. Although seemingly innocuous, an asymmetric appearance can negatively impact a patient’s psychological well-being and how they are perceived by others and themselves [1].
History of Cranioplasty
As medicine and surgical techniques have evolved, surgeons have had the goal of not only saving the patient’s life, but providing them a life of good quality, closest to their baseline as possible. From improving patient’s self-esteem, to social acceptance of ones who have had disfiguring surgeries such as a craniectomy that would leave part of the head depressed, to the cranioplasty reconstruction and hope for a fulfilling, productive life. For as long as craniectomy has been used to treat lesions of the cranium and cerebrum, surgeons have utilized cranioplasty techniques to re-establish the layer of protection afforded by the skull and mitigate cosmetic and neurological deficits [2]. Trephination or commonly known as a burr hole, is one of the first surgical procedures known to humanity, dating back to 9000 years ago. The Cranioplasty as such, was done using materials such as gold plates due to their high value rather than efficacy of the implant. Although banned by Hippocrates in 400 BC, surgeons renewed the procedure again the 16th century, and with advancements in medicine and technology it became more popular and considered safer during the 20th century [3]. From continuous use of gold plates in 1500s to canine cranium in the 1600s and the first autograft in the 1800s, even though they each had their cons and pros, they were the main surgical options available for surgeons, until the development of autograft [4]. Other animals such as eagle, goose, and ape have been used as xenografts by boiling the bones to sterilize and then placing it on undamaged dura [2]. Ever Since there was a rapid advancement in cranioplasty method development and improvement in patient survival until the current time.
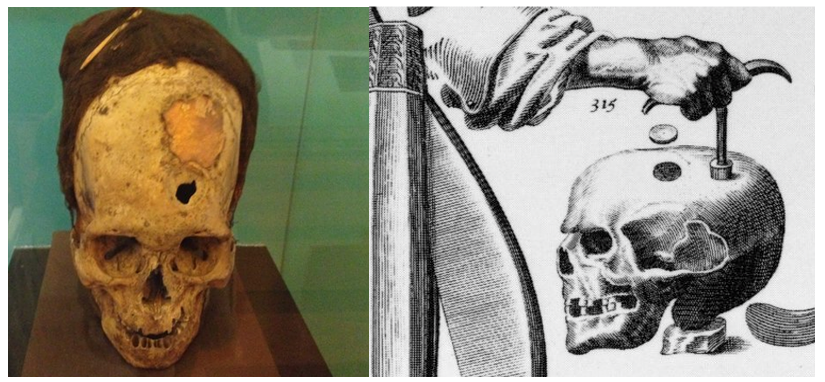
Figure 1: Showing an example of Trephination and cranioplasty with gold implant.
Charles G. Gross. A Hole in the Head: More Tales in the History of Neuroscience. The MIT Press. 2009. https://doi.org/10.7551/mitpress/7759.001.0001
Basic Principles of Cranioplasty
Cranioplasty restores the anatomical cranial architecture, thus returning the protective functions of the skull and improving cranial appearance and function post-craniectomy. Restoration of cranial architecture will also play a role in normalizing CSF flow dynamics and maintaining normal intracranial pressure [1,5]. For a cranial implant to achieve these goals, it must be fabricated and positioned in a way that restores the patient’s preoperative craniofacial architecture without impeding neurological function or implicating additional health risks. The ideal material for cranioplasty is thus lightweight, durable, easily fixable to the skull, osteoconductive, and malleable [1]. This article describes the evolution of cranioplasty materials and operative techniques through the present day. Basic principles and historical context are included to supplement the reader’s understanding of cranioplasty’s ultimate goals and ideals. The article aims to culminate in a comparative examination of current techniques that are reported to improve clinical outcomes. This will allow us to identify obstacles and limitations to modern cranioplasty and to identify opportunities to further develop the field of neuroplastic surgery.
Method
Search strategy for this article included using databases including PubMed, Google Scholar, and Web of Science. These databases were used to obtain scholarly articles and book chapters from credible scientific journals. The keywords used to search included cranioplasty, craniectomy, defect correction. There were no time or country limitations. Cases were mainly focused on cranial aspect of cranioplasty, the maxillofacial focused cases were eliminated from the search base.
Discussion
The Cranial Implant
When deciding the material of a cranial implant, the following properties must be considered: radiolucency, magnetic properties (should not be magnetic), cytotoxicity, resistance to infection, resorbability, tendency to erode or discolor surrounding tissue, malleability, heat conduction (should not dilate when subject to heat), durability, cost, and availability [6,7]. Types of implants include autologous, metal allografts, and custom cranial implants made from Titanium.
Autologous bone grafts are harvested from a broad range of areas in the patient’s body including but not limited to iliac crest and ribs to be surgically integrated into the cranial defect. The original skull removed during the patient’s craniectomy is often used as an autologous bone graft. Harvesting autografts from different body parts are unfavorable as they require secondary surgery to harvest and extensive effort to customize and maintain prior to cranioplasty. Extended time in cryopreservation has been identified as a risk factor for bone flap resorption [8]. Many autografts also have a high risk of resorption and consequent repeat surgery [6]. Kwarcinski et al. (2017) examined 10 different studies of 686 total patients that received autologous bone graft cranioplasty to determine an average infection rate of 10.50% for the procedure.
Metal allografts using titanium alloy mesh/plating are still widely used despite the existence of more ideal materials. Aside from lacking malleability, this option is affordable, durable, radiolucent, and bio-acceptable [6]. Kwarcinsiki et al. (2017) examined 10 studies of 1053 total patients that underwent cranioplasty with a titanium mesh or plate implant to determine an average infection rate of 8.01%.
Custom Cranial Implants (CCIs) are most commonly created using polymethylmethacrylate (PMMA) or polyether-ether-ketone (PEEK) due to their superior capacity to meet the aforementioned material requirements relative to alternatives [3,6]. Most recently, the use of cements such as calcium phosphate and calcium sulfate has been under investigation as well [9]. These synthetic polymers have been shown to adhere to the dura mater without reacting to other layers in animal models [6,10]. The advantages of PEEK are its strength, stiffness, durability over time, thermal nonconductivity, and rapid manufacturing [11]. There are, however, concerns regarding the anatomical integration potential of PEEK implants due to the material’s hydrophobic properties [12]. PMMA shares the same advantages as PEEK, but has a more textured surface, which promotes soft tissue adherence, thus improving implant stability [11]. CCIs are preferred to autologous bone grafts because they eliminate the risk of resorption and warping. CCIs are also preferred to titanium mesh/plating due to their ability to provide full-thickness reconstructions and eliminate the risks associated with leaving dead space [13]. Kwarcinski et al. (2017) examined 8 studies with a total of 152 patients that underwent cranioplasty with CCIs fabricated using PEEK to determine an average infection rate of 7. 89%. The same group examined 8 studies with a total of 274 patients that underwent cranioplasty with prefabricated PMMA CCIs and determined an average infection rate of 6.99%.
Current Techniques to Improve Clinical Outcomes
Scalp Anatomy
The scalp consists of five layers that can be easily remembered in order from superficial too deep with the acronym, “S-C-A-L-P”: Skin, subcutaneous connective tissue, galea aponeurotic fascia, loose areolar tissue, pericranium [14].
Axial-pattern scalp flaps are usually designed around five major vascularization of the scalp, two of which are branches of the Internal Carotid Artery (ICA) and three of which are branches of the External Carotid Artery (ECA). The ICA-associated arteries are the supratrochlear and supraorbital arteries, which are terminal branches of the ophthalmic artery, the first intracranial branch of the ICA. The ECA-associated arteries include the superficial temporal, posterior auricular, and occipital arteries, which are all terminal branches of the ECA [15]. Conventionally, a flap is identified by its supplying artery.
Principles and Considerations for Incision Planning
It is paramount for the neurosurgeon to deeply consider the design of a scalp flap when planning a cranioplasty operation. The flap must be allowed to rotate in such a way that sufficiently exposes the cranial defect. Adequate blood supply of the scalp flap is also of critical importance because an incision that cuts throw the blood supply can lead to local tissue necrosis, therefore prevent the healing of the surgical site. An incision should be made to produce an axial-pattern pedicle graft that incorporates a direct cutaneous artery and vein into its base [16]. Planning around major arteries of the scalp ensures adequate perfusion and cosmetically optimal wound healing. Incisions should also be planned to avoid damaging these vessels and other neurovascular structures [17]
Poor scalp vascularization can delay wound healing and increase the risk of incisional scalp dehiscence, infection, and repeated surgery. Factors that may contribute to excessive scalp thinning or poor vascularization include previous or active irradiation therapy, chemotherapy, smoking, advanced age, previous cranial surgery, and diabetes mellitus [16].
Component Separation of the Scalp
Component separation is achieved by dissecting the retaining ligaments that extend from the galea aponeurosis to the pericranium. These ligaments will appear as immobile, fibrous adhesions and should be dissected between the galeal fascia and the areolar connective tissue to separate the superficial three layers of the scalp from the deeper two [18]. The described technique has been shown to enable an additional 1-2 centimeter (cm)of scalp mobility, which is necessary to accommodate the additional convexity of preferred CCIs [18]. Scalp flexibility is made inherently possible by the scalp’s anatomy - the skin, subcutaneous connective tissue, and galea aponeurosis are tightly bound and form a vascular network that runs parallel to that of the pericranium. Since the loose areolar tissue that binds the galea and pericranium is avascular, it can easily be dissected without sacrificing dermal, subdermal, or calvarial perfusion [18]. Component separation is important for the neurosurgeon to have a tension-free closure, reducing a patient’s risk for incisional scalp dehiscence and infection.
The Pericranial-Onlay Technique
The pericranial-onlay is a cranioplasty technique that was developed to reduce the high complication rates associated with placing autologous bone flaps and alloplastic implants directly over the dura or dural substitute [13].
After making an incision along the previous surgical wound and elevating the scalp flap, fine-needlepoint electrocautery is used to perform a component separation of the scalp on the loose areolar plane. Having elevated a fasciocutaneous scalp flap consisting only of galea fascia and skin, the surgeons are left with a vascularized pericranium covering the epidural space [19]. To minimize operative duration, it is best practice to have one team complete the scalp dissection while another prepares the cranial implant for placement by shaving it down to fit within the cranial defect and adding titanium plates and screws. If the cranioplasty is a revision surgery due to bone flap infection, the edges of the cranial defect must be adequately debrideded prior to implant placement. The cranial implant is then properly positioned within the defect over the pericranial-onlay and fastened to the surrounding skull [19].
Potential Risks and Complications
Cranioplasty, like any other surgical procedure, has known complications for which neurosurgeons must be prepared to manage. Common complications include infection, seizures, epidural and subdural hematoma and hydrocephalus, all placing the patient at risk for revision surgery [19]. Furthermore, patients who are active smokers are at higher risk for these complications given the delayed healing effects of nicotine and cigarette smoke [20]. While adequate healing is critical to the success of the cranioplasty,post-operative bone flap resorption places the patient at risk for revision surgery and/or the inability to use the autograft at the time of surgery. Resorption results in a deterioration and shrinkage of an autologous bone graft and thus limits the integrity of the skull at reconstruction due to an inadequately fitting implant [21]. Incomplete reconstruction leaves the brain more vulnerable to direct external injury from impact which may subsequently require another surgery, thus increasing all risks inherent with surgery. It has been shown by Kim et al. (2015) that patients who underwent primary craniectomy for Traumatic Brain Injury (TBI) and patients that have more fractures/fragments in the reinserted bone flap are at significantly higher risk for bone flap resorption post-cranioplasty. Young age, bone flap fragmentation, and long storage time have been identified as significant risk factors for bone flap resorption [8].
Post-operative wound dehiscence at the layers of the scalp or galea aponeurotica can be a devastating complication requiring explant, prolonged antibiotic courses, and delayed reconstruction. Multiple surgeries may lead to poor vascularization which can increase the risk of wound dehiscence. In general, cranial implants increase the risk of incisional scalp dehiscence. As explained by Blake (1994) Poorly fitted cranial implants may also erode the scalp.
Minimizing areas of dead space beneath the scalp and cranial implant is critical when closing the surgical incision. Failure to do so may result in the collection of blood or fluid (hematoma or seroma), placing the implant and sclap under unnecessary tension and providing a nidus for infection [22]. Such risks should also be considered when using a mesh plate for cranioplasty. The mesh graft’s pores may invite subgaleal or epidural accumulation of blood or fluid [22].
The risk of infection is inherent to all invasive procedures and must be mitigated through diligent maintenance of a sterile surgical field. Factors that have been identified to increase infection risk in cranioplasty operations include prolonged operative duration (>200 minutes), number of previous operations (three or more times), previous infection, and latency period between tissue removal and implant insertion [12]. Patients with bifrontal repair requirements and convexity cranioplasty have been shown to have a higher risk of infection, possibly due to the involvement of frontal sinus [11]. Interestingly graft material did not show a significant correlation between seizure and infection rate [23].
Improper placement of a cranial implant may have a negative impact on a patient’s neurological functioning. The risks of implant misplacement are particularly elevated during orbital bandeau reconstruction due to this region’s proximity to frontal sinus mucous membranes and cranial nerves III and VI - the oculomotor and abducens nerves. Poor implant placement relative to the frontal sinus mucous membranes can result in mucocele formation, breakdown of alloplastic materials, and subsequent infection [8]. Poor implant placement relative to the orbital roof, apex, or medial wall can result in compromised neurovasculature, compromised visual acuity, or external ophthalmoplegia [8].
Conclusion
Future Directions of Cranioplasty
Recent advances in Computer-Aided Design and Manufacturing (CAD/CAM) technologies have paved the way for feasible, patient-specific, and regenerative new solutions for cranial defects. Rapid progress in 3-D printing technology that uses synthetic polymers like PMMA and PEEK has become more popular for the fabrication of patient-specific cranial implants.
Moreover, an increasing number of studies suggest that CCIs exhibit an improved outcome profile for large cranial defect reconstruction. CCIs also reduce complication rates across the board by eliminating the risk of bone flap resorption, decreasing the risk of developing microbial infections, and decreasing the risk of blood and fluid collection in dead spaces. These improvements sway the long-term cost differential in favor of synthetic CCIs over autologous bone grafts and titanium allografts. In addition to their ideal material properties, synthetic CCIs can also be fabricated to fit a wide range of technologies for postoperative monitoring and, in some cases, further treatment.
Paper limitations: This is a retrospective paper, using the information available based on the research. Specific diseases such as connective tissue or more general hypertension and diabetes were not used as specific comparisons. The long-term followup or a specific short-term follow-up was not separately assessed.
References
- Piazza M, Grady MS. Cranioplasty. Neurosurgery Clinics of North America, 2017; 28: 257-265. https://doi.org/10.1016/j.nec.2016.11.008.
- Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis, Neurosurgical Focus FOC, 2014; 36(4). https://doi.org/10.3171/2014.2.FOCUS13561.
- Durand JL, Renier D, Marchac D: [The history of cranioplasty]. Annales de Chirurgie Plastique et Esthetique. 1997; 42: 75-83. https://dx.doi.org/10.1016/j.injury.2011.02.004.
- Alkhaibary A, Alharbi A, Alnefaie N, Oqalaa Almubarak A, Aloraidi A, Khairy S. Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects, and Complications. World Neurosurgery, 2020; 139: 445-452. https://doi.org/10.1016/j.wneu.2020.04.211..
- Chen X, Xu L, Li X, Egger J. Computer-aided implant design for the restoration of cranial defects. Scientific Reports, 2017; 7: 1. https://doi.org/10.1038/s41598-017-04454-6.
- Aydin S, Kucukyuruk B, Abuzayed B, Aydin S, Sanus GZ. Cranioplasty: Review of materials and techniques. Journal of Neurosciences, 2011; 2: 162-167. https://dx.doi.org/10.4103/0976-3147.83584
- Blake DP. The Use of Synthetics in Cranioplasty: A Clinical Review. Military Medicine, 1994; 159: 466-469. https://doi.org/10.1093/milmed/159.6.466.
- Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scandinavian Journal. of Trauma, Resuscitation and Emergency Medicine, 2015; 23: 75. https//doi.org/10.1186/s13049-015-0155-6.
- Drosos GI, Babourda E, Magnissalis EA, Giatromanolaki A, Kazakos K, Verettas DA. Mechanical characterization of bone graft substitute ceramic cements. 2012; 43: 266-271. https://doi.org/10.1016/j.injury.2011.02.004.
- Shay T, Mitchell KA, Belzberg M, et al. Translucent Customized Cranial Implants Made of Clear Polymethylmethacrylate: An Early Outcome Analysis of 55 Consecutive Cranioplasty Cases, 2020; 85: 27. https://doi.org/10.1097/SAP.0000000000002441.
- Kwarcinski J, Boughton P, Ruys A, Doolan A, Van Gelder J. Cranioplasty and Craniofacial Reconstruction: A Review of Implant Material, Manufacturing Method and Infection Risk. Applied Sciences, 2017; 7. https://doi.org/10.3390/app7030276.
- Alvi S, Jenzer AC. Scalp Reconstruction.: StatPearls [Internet]. StatPearl, Treasure Island (FL), 2024. https://pubmed.ncbi.nlm.nih.gov/30969610/.
- Tajran J, Gosman AA: Anatomy, Head and Neck, Scalp. 2023 Jul 24. In. StatPearls [Internet, Treasure Island (FL): StatPearls Publishing, 2024. https://pubmed.ncbi.nlm.nih.gov/31855392/.
- Shafiuzama M, Sabarish Babu MS, Mohamed A, Sankar P, Sindhu GN, Hemalatha S, et al. Axial pattern flaps, using the deep circumflex iliac artery, superficial brachial and cranial superficial epigastric direct cutaneous arteries in the dog. Iranian Journal of Veterinary Research. 2017, 18:216-220. https://pubmed.ncbi.nlm.nih.gov/29163653/.
- Weitz J, Spaas C, Wolff K-D, Meyer B, Shiban E, Ritschl LM. A Standard Algorithm for Reconstruction of Scalp Defects with Simultaneous Free Flaps in an Interdisciplinary Two-Team Approach. Front oncology, 2019; 9: 1130. https://dx.doi.org/10.3389/fonc.2019.01130.
- Ibrahim Z, Santiago GF, Huang J, Manson PN, Gordon CR. Algorithmic Approach to Overcome Scalp Deficiency in the Setting of Secondary Cranial Reconstruction. The. Journal of Craniofacial Surgery, 2016; 27: 229-233. https://doi.org/10.1097/SCS.0000000000002289.
- Gordon CR, Fisher M, Liauw J, et al. Multidisciplinary approach for improved outcomes in secondary cranial reconstruction: Introducing the pericranial-onlay cranioplasty technique. Neurosurgery, 2014; 10 Suppl 2: 179-189. https://dx.doi.org/10.1227/NEU.0000000000000296.
- Zanaty M, Chalouhi N, Starke RM, et al. Complications following cranioplasty: Incidence and predictors in 348 cases. Journal of Neurosurgery JNS, 2015; 123: 182-188. https://doi.org/10.3171/2014.9.JNS14405.
- Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg, 2010; 112: 1120-1124. https://dx.doi.org/10.3171/2009.6.JNS09133.
- Kim JS, Cheong JH, Ryu JI, Kim JM, Kim CH. Bone Flap Resorption Following Cranioplasty after Decompressive Craniectomy: Preliminary Report. Korean Journal of Neurotrauma, 2015; 11: 1-5. https://doi.org/10.13004/kjnt.2015.11.1.1.
- Bonda D. J., Manjila S., Selman W. R., & Dean D.: The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery, 2015; 5: 814-824. https://dx.doi.org/10.1227/NEU.0000000000000899.
- Calamia JR. Advances in computer-aided design and computer-aided manufacture technology. J Philipp Dent Assoc, 1996; 48: 31-40. https://pubmed.ncbi.nlm.nih.gov/9462062/.
- Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy:analysis of 62 cases. Neurosurg Focus, 2009; 26(6): E9. https://pubmed.ncbi.nlm.nih.gov/9768140/.

