The Effectiveness of HPV Vaccination Programs in Reducing the Incidence of Cervical Cancer: A Literature Review
Asma Marwan Al-Rawi1,* and Laila Yahya Al-Hubaishi2
1OBGYN PGY-1 at Dubai Health, United Arab Emirates (UAE)
2Consultant Obgyn at Dubai Health, United Arab Emirates (UAE)
Received Date: 17/07/2024; Published Date: 14/10/2024
*Corresponding author: Asma Marwan Al-Rawi, OBGYN PGY-1 at Dubai Health, United Arab Emirates
Abstract
Cervical cancer is caused by the HPV virus in a sexually active person. Cervical cancer staging is the most prognostic factor, followed by nodal status, tumor volume, depth of cervical stromal invasion, and lymph vascular space invasion. HPV infection is associated with most cervical cancer cases, with HPV-16 and -18 identified as the most carcinogenic subtypes, accounting for over 50% and 10% of cases, respectively. Cervical cancer staging is the most prognostic factor, followed by nodal status, tumor volume, depth of cervical stromal invasion, and lymph vascular space invasion. Most types of cancer have stages IIV. However, there are some types of cancer, including cervical cancer, which has stages 0 to IV. Key prevention initiatives include completing the recommended vaccination series, standardized screening, and education about contributing factors to encourage avoidance of associated risks. Multiple studies have demonstrated that all three vaccines exhibit excellent safety and tolerance in different age groups. A 10-year follow-up study showed that Gardasil is immunogenic, clinically effective, and generally well-tolerated in preadolescents and adolescents. Furthermore, cervix and Gardasil 9 demonstrate great tolerance and antibody sustenance after vaccination for up to 9.4 years and 6 years, respectively. Headache and fatigue are the most common Cervarix-related systemic AE. Gardasil and Gardasil 9 recipients may also have general symptoms, but no increased risk of systemic symptoms was evident in their recipients.
Cervical Cancer
Introduction
Introduction
Cancer of the cervix is predominantly caused by persistent human papillomavirus (HPV) infections [1]. Out of 200 identified HPV types, 12 have been designated as carcinogenic by the International Agency for Research on Cancer, with HPV-16 accounting for 50% and HPV-18 accounting for 10% of cervical cancer cases, respectively [2]. Infection with one of these two strains of HPV accounts for a 435-fold and 248-fold increase in cancer risk, respectively, as compared with an uninfected individual [3]. Persistent viral infection with high-risk HPV genotypes is the causative agent and can be detected in 99.7 % of patients with cervical cancer worldwide [4]. HPV infection is sexually transmitted and roughly 80% of women will be infected at some point in their lifetime, many by the age of 45. HPV infection is often contracted during adolescence and early adulthood and because the infection is asymptomatic it may take 10 to 15 years to manifest changes in the cervix [5]. Since the introduction of HPV vaccines, cervical cancer rates have decreased by 1% to 1.9% annually [3].
Incidence and Mortality
Cervical cancer is the third leading malignancy among women after breast and colorectal cancers worldwide, with 569,000 new cases each year [6]. Over 13,000 new cases and 4,100 cervical cancer deaths are estimated to occur in 2018. Cervical cancer occurs at disproportionately higher rates in less developed countries, likely because of reduced access to screening and the high cost of HPV vaccines [7]. HPV infection is associated with most cervical cancer cases, with HPV-16 and -18 identified as the most carcinogenic subtypes, accounting for over 50% and 10% of cases, respectively [8]. Additional subtypes have been identified, though less frequently, in cervical cancer cases. These include HPV-31, -33, and -45, each associated with approximately 5% of cases, HPV-52 with 3%, and HPV-35 and-58 each with 2% [3]. It is important to note that, while rare, HPV-negative cases have been identified; in one study such cases were associated with adenocarcinomas, advanced stage presentation, and poorer disease-free survival when compared with HPV-positive cases [9].
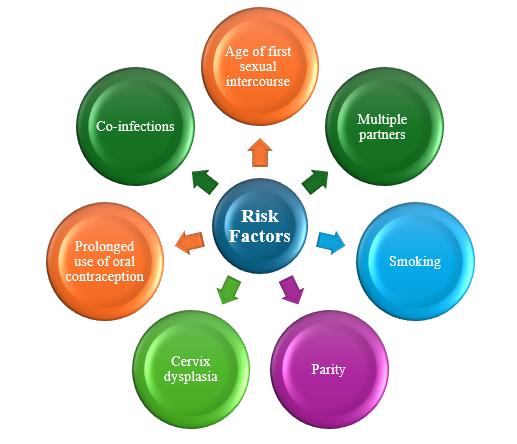
Risk Factors
Risk factors of cervical cancer include both behavioral and infectious contributors [10]. Behavioral contributors include sexual activity and lifestyle factors (Table 1). Cervical cancer is caused by the HPV virus in a sexually active person. It is not transmitted genetically, and diet has no role in preventing cervical cancer. The age of first sexual intercourse increases the risk for cervical cancer, with the first sexual encounter at a younger age or proximity to menarche increasing the risk [11].
Criteria of Diagnosis
- Clinical Presentation
While early-stage cervical cancer is often asymptomatic, the most common symptoms at presentation are irregular or heavy vaginal bleeding, particularly following intercourse [12]. Some women may present with a vaginal discharge that may be watery, mucoid, or purulent and malodorous. For advanced disease, patients may present with pelvic or lower back pain that may radiate along the posterior side of the lower extremities [3].
- Physical Examination
A pelvic examination should be performed in women with symptoms suspicious of cervical cancer [13]. Speculum examination may reveal a normal cervix or a visible lesion. Large tumors may appear to replace the cervix entirely. Any questionable lesion should be biopsied. A thorough pelvic examination includes a rectovaginal examination to assess the tumor size and vaginal or parametrial involvement [14]. Cervical cancer is diagnosed based on the histologic evaluation of a cervical biopsy. The two most common histopathologic types of cervical cancer include squamous cell carcinoma (up to 85% of cases) and adenocarcinoma (up to 25%), including adenosquamous, and another histologist (6%) [15]. Additional yet uncommon histologists include small cell or neuroendocrine, clear cell, and serous papillary. Nonsquamous presentations are associated with a poorer prognosis [3].
Cervical Cancer Staging
Cervical cancer staging is the most prognostic factor, followed by nodal status, tumor volume, depth of cervical stromal invasion, and lymph vascular space invasion [16]. The prognosis is worse for women with involved pelvic or para-aortic nodes. The International Federation of Gynecology and Obstetrics is the most used staging for cervical cancer (Table 2), [17].
Table 2: International Federation of Gynecology and Obstetrics (FIGO) Staging.
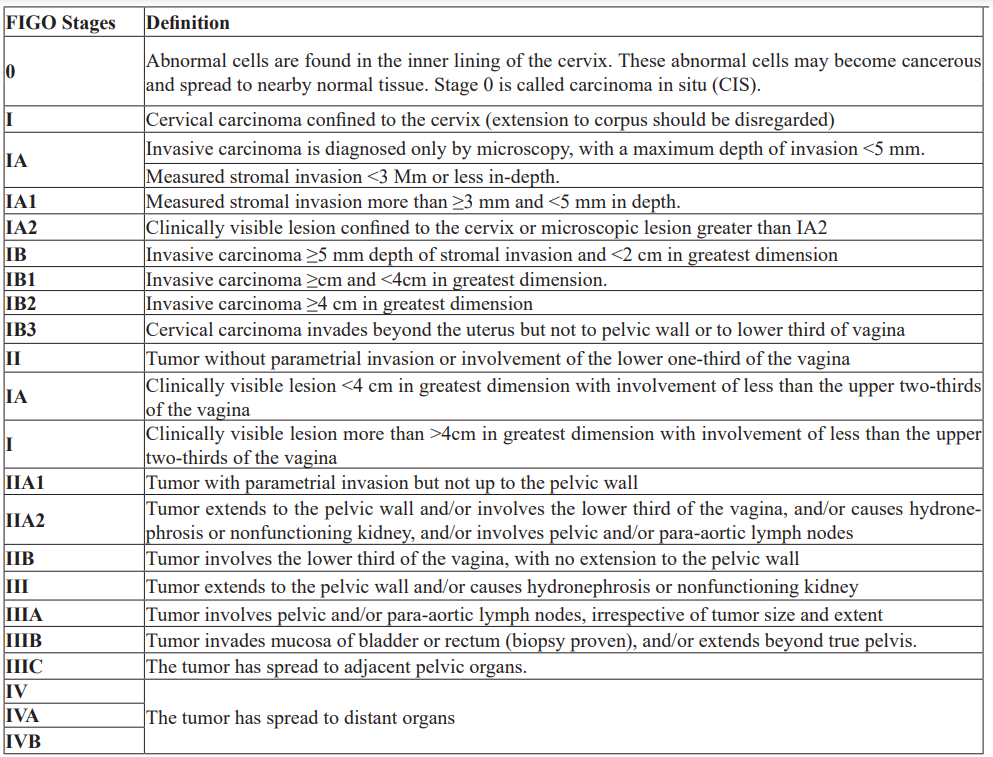
Most types of cancer have stages IIV. However, there are some types of cancer, including cervical cancer, which has stage 0 to IV [3]. Stage 0: when abnormal cells are found in the inner lining of the cervix. Stage 0 is also called carcinoma in situ; Stage I: when the cancer is confined in the cervix only; Stage II: when cancer has spread beyond the cervix but has not spread to the pelvic wall or the lower third of the vagina; Stage III: when cancer has spread to the lower third of the vagina and/ or may have spread to the pelvic wall, and/or has caused kidney injury; Stage IV: when cancer has spread to the bladder, rectum, or other parts of the body [18].
Treatment Options
- Surgical Treatment
Surgery is part of the treatment for many cases of cervical cancer [19]. For small precancerous lesions (carcinoma in situ) or cervical cancer contained in the cervix (stage I), cryosurgery (cryotherapy), laser surgery, loop electrosurgical excision procedure, conization, hysterectomy, and bilateral salpingo-oophorectomy may be used [20]. For larger cervical cancer lesions (usually up to 45 cm in width), trachelectomy (a fertility-sparing procedure) and radical hysterectomy may be used [21]. These surgeries can be performed with a laparoscope, using a robotic machine, or with a larger abdominal incision (laparotomy), [22].
- Radiation
For very large lesions (larger than 4 cm) or metastatic cervical cancer, radiation with concurrent chemotherapy is usually the standard of care for primary treatment [23]. The type of chemotherapy used will be discussed in the next section. Radiation therapy may be used instead of surgery, or as an adjuvant therapy following surgery [24]. Three types of RT may be used to treat cervical cancer: external RT, including intensity-modulated radiotherapy (IMRT), and internal RT (brachytherapy), [25].
- Chemotherapy
Chemotherapy has typically been used for advanced or recurrent diseases that can no longer be treated or managed by surgery or RT [26]. Today, chemotherapy has taken a much bigger role as part of definitive treatment for cervical cancer [27]. For newly diagnosed cervical cancer stage I-IB2 or higher, Cisplatin or Cisplatin in combination with Fluorouracil chemotherapy can be given along with RT as a radiosensitizer to help the radiation work better [20]. Among the chemotherapy agents, Cisplatin, Paclitaxel, and Carboplatin have shown the most consistent activity as single agents [28].
- Immunotherapy
Immunotherapy uses medicines that stimulate the one’s own immune system to recognize and destroy cancer cells [29]. An important part of the immune system is its ability to use molecules, also known as “checkpoints,” to turn on (or off) to activate an immune response [30]. Cancer cells often use these checkpoints to avoid being attacked by the immune system; however, newer drugs have been able to target these checkpoints to help fight cancer [3].
Prevention
Cervical cancer is a highly preventable disease with declining incidence because of effective screening and vaccination to prevent the most carcinogenic strains of HPV [10]. Key prevention initiatives include completing the recommended vaccination series, standardized screening, and education about contributing factors to encourage avoidance of associated risks. Condom use is reported as approximately 70% effective in reducing the transmission of HPV [3].
- Cervical Screening
Regardless of vaccination status, consistent cervical cancer screening is recommended beginning at age 21 in the US [31]. Papanicolaou cytology (Pap) tests are the current standard for screening. Starting at age 30, women should undergo a good women/pelvic exam annually, Pap test every 3 years, with HPV co-testing every 5 years until age 65. Women who are at high risk of cervical cancer should be tested often as per the recommendation of the healthcare team [32]. The Pap smear has a sensitivity of 55.4% and HPV co-testing has a sensitivity of 94.6% [33]. In addition to the cytology testing provided by Pap tests, HPV assays have also been indicated as sensitive, particularly in the evaluation of high-risk strains and detection of cervical intraepithelial neoplasia 2+ and 3+ [34].
- HPV Vaccination
A 9-valent HPV vaccine (Gardasil9) covering HPV strains 6, 11, 16, 18, 31, 33, 45, 52, and 58, received US Food and Drug Administration approval in December 2014 and is now the only available HPV vaccine in the United States (US) [35]. HPV vaccination is recommended to start at age 11 or 12 years but may be administered as early as age 9, and through age 26 if not previously vaccinated [36]. The vaccine may be administered in two doses to individuals who receive them 6 to 12 months apart before their 15th birthday [37], or in three doses for individuals beginning the series at 15 years of age or older, for immune-compromised individuals aged 9 to 26, or for those who receive the doses less than 5 months apart [3].
Human Papillomavirus (HPV)
Introduction
Human papillomavirus (HPV), one of the most common sexually transmitted viral infections worldwide, is the leading cause of cervical cancer, which ranks fourth globally in incidence and mortality rates [38]. However, cancer but also with anal, oropharynx, penile, vaginal, and vulvar cancer [39]. Moreover, the rates of HPV infection have increased over time. There are approximately 200 HPV types identified, with 40 of them known to be sexually transmitted [4]. HPV is classified into high-risk types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82), and low-risk types (HPV 11, 40, 42, 43, 44, 53, 54, 61, 72, 73, and 81), [40] The most detected HPV types, in almost 70% of cancers, are HPV 16 and 18 [41]. HPVs infect and replicate in the mucosal and cutaneous epithelia of their hosts [42]. These stratified epithelia contain a basal layer of self-renewing cells that divide symmetrically to replenish the basal layer, and asymmetrically to generate daughter cells that make up the differentiated layers of the tissue [43]. The HPV life cycle takes advantage of this process by establishing a reservoir of persistent infection in the self-renewing basal cells, and only generating virion particles in the terminally differentiated cells [42]. Viruses are released into the environment in squames (dead cells) that are sloughed from the surface of the epithelium. Different HPV types infect diverse anatomical regions of the cutaneous or mucosal epithelia, but they all have a similar differentiation-dependent life cycle (Figure 1), [44].
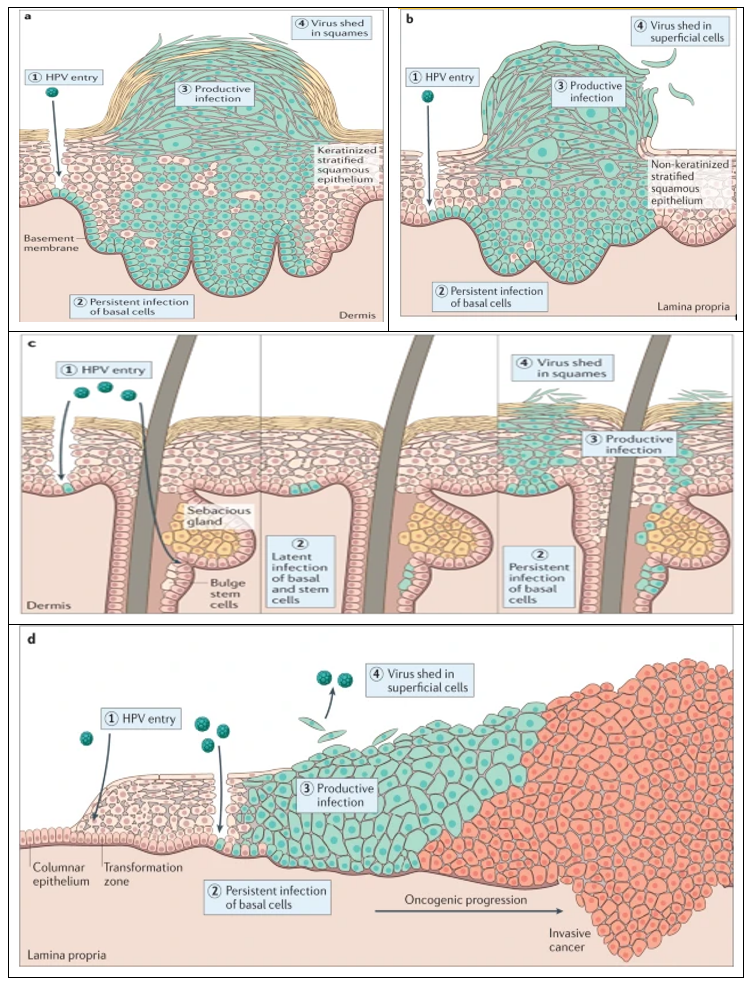
Figure 1: Human papillomavirus infectious cycles in stratified epithelia of different host tissues. common skin wart, often caused by Mupapillomavirus infection (panel a); anogenital wart infected with low-risk Alpha papillomaviruses (panel b); infection of the skin and hair follicles by Beta papillomavirus (panel c); cervical infection by high-risk Alpha papillomaviruses (panel d), [44].
Epidemiology of HPV
The National HPV Center reports overall fewer cases of HPV incidence in the MENA region compared to other regions globally, but at a lower age standardization rate [45]. The World Health Organization's vision in 2018 was to decrease HPV prevalence globally with the introduction of HPV vaccines into national vaccination programs [46]. However, because of the stigma related to HPV as a sexually transmitted infection, most of the conservative countries in the MENA region have yet to introduce HPV vaccination programs [47]. Of the 27 countries, Turkey has the only organized screening program, which it has had since 2013. Some countries, such as Algeria, Morocco, and Qatar, have implemented vaccination programs within their existing health programs but within opportunistic settings [48]. The only two countries that have the HPV vaccine embedded in their health programs are Libya and the United Arab Emirates (UAE), [49]. Although most of the countries in the MENA region have a low incidence rate of HPV infection, the coverage of the reported cases is typically based on small sample sizes, which underestimates or overestimates the actual number of cases [50]. One review that examined the effectiveness of screening and vaccination programs in the MENA region showed that 70% vaccination coverage could prevent 180,000 cases of cancer in the MENA region [39].
Mechanisms of Vaccinations
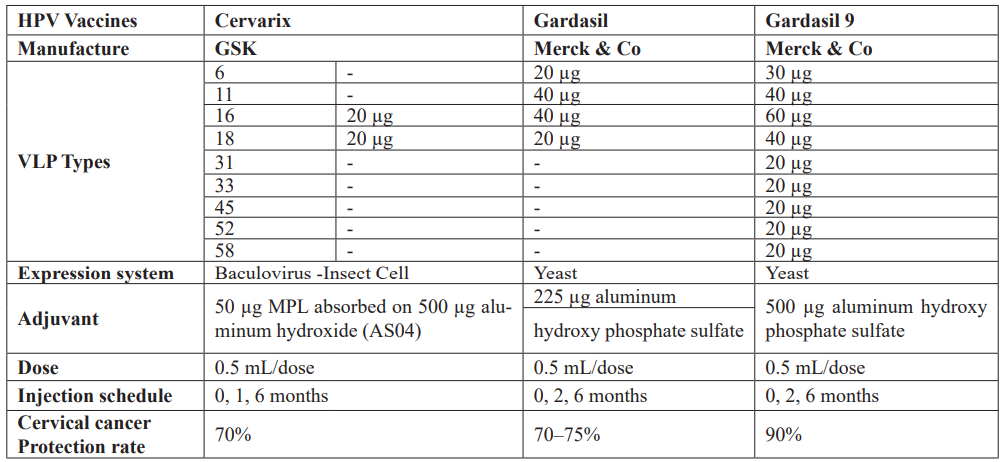
The licensed HPV vaccines are developed based on a virus-like particle (VLP) of the major papillomavirus capsid protein L1 [51]. Since VLPs are merely proteins and do not contain a viral genome, these are considered non-infectious and non-oncogenic and thus are safer than HPV-attenuated vaccines [52]. VLPs can be produced in bacteria, yeast, or insect cells. The cervix comprises HPV16 and 18 VLPs, monophosphorylate lipid A (MPL), and aluminum hydroxide (together called adjuvant system 04, AS04) as adjuvant [53]. MPL is a toll/like receptor 4 (TLR4) agonist that can induce high levels of antibodies as compared to Gardasil and Gardasil 9, both of which contain only aluminum hydroxide as an adjuvant and are produced in Saccharomyces cerevisiae yeast [52]. Gardasil contains VLPs against HPV6, 11, 16, and 18, while Gardasil 9 contains VLPs against HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 [54]. The HPV vaccines currently being produced are based on L1-VLPs, which only provide type-restricted immunity, neglecting many other oncogenic HPV genotypes [55]. Consequently, second-generation VLPs, such as L2-VLP and Chimeric L1-L2 VLP, are drawing a lot of attention for their broader genotype coverage [56]. In comparison to L1-VLP, the minor capsid protein L2 contains type-common epitopes that can provide broad cross-neutralizing antibody responses. Notably, Cervarix can confer a degree of cross-protection against some phylogenetically related types of HPV16 and 18 from the same phylogenetic cluster alpha-9 (HPV16-like: HPV31, 33, 35, 52, 58) and alpha-7 (HPV18-like: HPV39, 45, 59, 68) species groups, owing to its unique adjuvant systems [52].
Table 3: Comparison of HPV vaccines.
Vaccine Safety and Adverse Effects
Multiple studies have demonstrated that all three vaccines exhibit excellent safety and tolerance in different age groups [57]. A 10-year follow-up study showed that Gardasil is immunogenic, clinically effective, and generally well-tolerated in preadolescents and adolescents [58]. Furthermore, cervix and Gardasil 9 demonstrate great tolerance and antibody sustenance after vaccination for up to 9.4 years and 6 years, respectively [52]. The most frequent adverse effects (AEs) of Cervarix and gardasil were injection-site reactions, such as pain and swelling, possibly due to the VLP-related inflammation process [59]. Cervarix can also lead to systemic symptoms, such as fever, nausea, vomiting, dizziness, myalgia, and diarrhea [60]. Headache and fatigue are the most common Cervarix-related systemic AE, seen in approximately 50–60% of participants. Gardasil and Gardasil 9 recipients may also have general symptoms, but no increased risk of systemic symptoms was evident in their recipients [61].
Immunogenicity
Immunogenicity, a crucial factor influencing the effectiveness of vaccines, has been consistently demonstrated in HPV vaccines [62]. These vaccines elicit robust and sustained immune responses, surpassing the immunity achieved through natural infection [63]. Seroconversion, defined as developing specific antibodies against the viral antigen, was observed in nearly 100% of individuals vaccinated with the 3-dose series [64]. The titer of these antibodies increased after each dose and gradually declined over time following the completion of the vaccination course. Peak antibody titers induced by HPV vaccines are significantly higher than those generated after natural infection [65]. Immunological memory elicited by HPV vaccines results in persistent antibody titers, providing long-term protection [66]. Studies have demonstrated the stability of antibody levels over 9.4 years post-vaccination. This enduring immunity is attributed to the induction of memory B cells, which play a crucial role in maintaining immunological memory [67]. Titers exhibit an inverse relationship with age, with higher titers observed in individuals aged 9-26 years compared to older age groups [68]. Longitudinal evaluations of immune responses up to 24 months post-vaccination revealed a significant increase in geometric antibody titers (GMTs) for HPV type 16 (2.4-5.8-fold) and HPV type 18 (7.7-9.4-fold). These findings underscore the robust and sustained immunological response induced by HPV vaccines [69].
Conclusion
Cervical cancer is the third leading malignancy among women after breast and colorectal cancers worldwide, with 569,000 new cases each year. Cervical cancer occurs at disproportionately higher rates in less developed countries. HPV infection is associated with most cervical cancer cases, with HPV-16 and -18 identified as the most carcinogenic subtypes, accounting for over 50% and 10% of cases, respectively. Key prevention initiatives include completing the recommended vaccination series, standardized screening, and education about contributing factors to encourage avoidance of associated risks. Multiple studies have demonstrated that all three vaccines exhibit excellent safety and tolerance in different age groups. A 10-year follow-up study showed that Gardasil is immunogenic, clinically effective, and generally well-tolerated in preadolescents and adolescents. Furthermore, cervix and Gardasil 9 demonstrate great tolerance and antibody sustenance after vaccination for up to 9.4 years and 6 years, respectively. Gardasil and Gardasil 9 recipients may also have general symptoms, but no increased risk of systemic symptoms was evident in their recipients.
References
- Sammarco ML, Tamburro M, Pulliero A, Izzotti A, Ripabelli G. Human papillomavirus infections, cervical cancer and MicroRNAs: an overview and implications for public health. MicroRNA, 2020; 9(3): 174-186.
- Wall JA, Boitano TK, Massad LS, Huh WK. Preinvasive disease of the cervix. DiSaia and Creasman Clinical Gynecologic Oncology, 2023; 1-9.
- Johnson CA, James D, Marzan A, Armaos M. Cervical cancer: an overview of pathophysiology and management. InSeminars in oncology nursing, WB Saunders, 2019; 35(2): pp. 166-174).
- Okunade Human papillomavirus and cervical cancer. Journal of Obstetrics and Gynaecology, 2020; 40(5): 602-608.
- Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, et al. Human papillomavirus: Apprehending the link with carcinogenesis and unveiling new research avenues. International journal of oncology, 2018; 52(3): 637-655.
- Huo X, Zhou X, Peng P, Yu M, Zhang Y, Yang J, et al. Identification of a six-gene signature for predicting the overall survival of cervical cancer patients. OncoTargets and therapy, 2021; 809-822.
- Nguyen NY, Okeke E, Anglemyer A, Brock T. Identifying perceived barriers to human papillomavirus vaccination as a preventative strategy for cervical cancer in Nigeria. Annals of Global Health, 2020; 86(1).
- Hajek M, Sewell A, Kaech S, Burtness B, Yarbrough WG, Issaeva N. TRAF3/CYLD mutations identify a distinct subset of human papillomavirus‐associated head and neck squamous cell carcinoma. Cancer, 2017; 123(10): 1778-1790.
- Arezzo F, Cormio G, Loizzi V, Cazzato G, Cataldo V, Lombardi C, et al. HPV-negative cervical cancer: a narrative review. Diagnostics, 2021; 11(6): 952.
- Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chinese Journal of Cancer Research, 2020; 32(6): 720.
- Kashyap N, Krishnan N, Kaur S, Ghai S. Risk factors of cervical cancer: a case-control study. Asia-Pacific journal of oncology nursing, 2019; 6(3): 308-314.
- Olejniczak L, Zasowska-Nowak A. The management of vaginal bleeding in advanced cervical cancer. Medycyna Paliatywna/Palliative Medicine, 2023; 15(3): 93-99.
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. The Lancet, 2019; 393(10167): 169-182.
- Salvo G, Odetto D, Perrotta MC, Noll F, Perrotta M, Pareja R, et al. Measurement of tumor size in early cervical cancer: an ever-evolving paradigm. International Journal of Gynecologic Cancer, 2020; 30(8).
- Rozario SD, Silva IF, Koifman RJ, Silva IF. Characterization of women with cervical cancer assisted at Inca by histological type. Revista de saude publica, 2019; 53: 88.
- Santoro A, Inzani F, Angelico G, Arciuolo D, Bragantini E, Travaglino A, et al. Recent advances in cervical cancer management: a review on novel prognostic factors in primary and recurrent tumors. Cancers, 2023; 15(4): 1137.
- Olawaiye AB, Baker TP, Washington MK, Mutch DG. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA: a cancer journal for clinicians, 2021; 71(4): 287-298.
- Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. International journal of gynecology & obstetrics, 2018; 143: 22-36.
- Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 2017; 28: 72-83.
- Jasrotia R, Dhanjal DS, Bhardwaj S, Sharma P, Chopra C, Singh R, et al. Nanotechnology based vaccines: Cervical cancer management and perspectives. Journal of Drug Delivery Science and Technology, 2022; 71: 103351.
- Costales A, Michener C, Escobar-Rodriguez PF. Radical trachelectomy for early-stage cervical cancer. Current Treatment Options in Oncology, 2018; 19: 1-2.
- Pascual M, Salvans S, Pera M. Laparoscopic colorectal surgery: current status and implementation of the latest technological innovations. World journal of gastroenterology, 2016; 22(2): 704.
- Zhou Y, Rassy E, Coutte A, Achkar S, Espenel S, Genestie C, et al. Current standards in the management of early and locally advanced cervical cancer: update on the benefit of neoadjuvant/adjuvant strategies. Cancers, 2022; 14(10): 2449.
- Jabo B, Lin AC, Aljehani MA, Ji L, Morgan JW, Selleck MJ, et al. Impact of breast reconstruction on time to definitive surgical treatment, adjuvant therapy, and breast cancer outcomes. Annals of Surgical Oncology, 2018; 25: 3096-3105.
- Chino J, Annunziata CM, Beriwal S, Bradfield L, Erickson BA, Fields EC, et al. Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline. Practical radiation oncology, 2020; 10(4): 220-234.
- Lee AW, Ng WT, Chan JY, Corry J, Mäkitie A, Mendenhall WM, et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer treatment reviews, 2019; 79: 101890.
- Burmeister CA, Khan SF, Schäfer G, Mbatani N, Adams T, Moodley J, et al. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Research, 2022; 13: 200238.
- Brown A, Kumar S, Tchounwou PB. Cisplatin-based chemotherapy of human cancers. Journal of cancer science & therapy, 2019; 11(4).
- Dhar R, Seethy A, Singh S, Pethusamy K, Srivastava T, Talukdar J, et al. Cancer immunotherapy: Recent advances and challenges. Journal of Cancer Research and Therapeutics, 2021; 17(4): 834-844.
- Brom VC, Burger C, Wirtz DC, Schildberg FA. The role of immune checkpoint molecules on macrophages in cancer, infection, and autoimmune pathologies. Frontiers in Immunology, 2022; 13: 837645.
- Fontham ET, Wolf AM, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA: a cancer journal for clinicians, 2020; 70(5): 321-346.
- Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Jama, 2018; 320(7): 674-686.
- Pruski D, Millert-Kalinska S, Lewek A, Kedzia W. Sensitivity and specificity of HR HPV E6/E7 mRNA test in detecting cervical squamous intraepithelial lesion and cervical cancer. Ginekologia polska, 2019; 90(2): 66-71.
- Sambursky JA, Terlizzi JP, Goldstone SE. Testing for human papillomavirus strains 16 and 18 helps predict the presence of anal high-grade squamous intraepithelial lesions. Diseases of the Colon & Rectum, 2018; 61(12): 1364-1371.
- Signorelli C, Odone A, Ciorba V, Cella P, Audisio RA, Lombardi A, et al. Human papillomavirus 9-valent vaccine for cancer prevention: a systematic review of the available evidence. Epidemiology & Infection, 2017; 145(10): 1962-1982.
- Saslow D, Andrews KS, Manassaram‐Baptiste D, Smith RA, Fontham ET, American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA: a cancer journal for clinicians, 2020; 70(4): 274-280.
- Liang JL. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports, 2018; 67.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 2018; 68(6): 394-424.
- Obeid DA, Almatrrouk SA, Alfageeh MB, Al-Ahdal MN, Alhamlan FS. Human papillomavirus epidemiology in populations with normal or abnormal cervical cytology or cervical cancer in the Middle East and North Africa: A systematic review and meta-analysis. Journal of Infection and Public Health, 2020; 13(9): 1304-1313.
- Okoye JO, Ofodile CA, Adeleke OK, Obioma O. Prevalence of high-risk HPV genotypes in sub-Saharan Africa according to HIV status: a 20-year systematic review. Epidemiology and Health, 2021; 43.
- Petrelli F, De Santi G, Rampulla V, Ghidini A, Mercurio P, Mariani M, et al. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: a systematic review and meta-analysis. Journal of Cancer Research and Clinical Oncology, 2021; 147(10): 3011-3023.
- Della Fera AN, Warburton A, Coursey TL, Khurana S, McBride AA. Persistent human papillomavirus infection. Viruses, 2021; 13(2): 321.
- Cockburn K, Annusver K, Gonzalez DG, Ganesan S, May DP, Mesa KR, et al. Gradual differentiation uncoupled from cell cycle exit generates heterogeneity in the epidermal stem cell layer. Nature Cell Biology, 2022; 24(12): 1692-1700.
- McBride Human papillomaviruses: diversity, infection and host interactions. Nature Reviews Microbiology, 2022; 20(2): 95-108.
- Momenimovahed Z, Mazidimoradi A, Maroofi P, Allahqoli L, Salehiniya H, Alkatout I. Global, regional and national burden, incidence, and mortality of cervical cancer. Cancer Reports, 2023; 6(3): e1756.
- Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Preventive medicine, 2021; 144: 106399.
- Varer Akpinar C, Alanya Tosun S. Knowledge and perceptions regarding Human Papillomavirus (HPV) and willingness to receive HPV vaccination among university students in a north-eastern city in Turkey. BMC Women's Health, 2023; 23(1): 299.
- Hakimi S, Lami F, Allahqoli L, Alkatout I. Barriers to the HPV vaccination program in the Eastern Mediterranean region: a narrative review. Journal of the Turkish German Gynecological Association, 2023; 24(1): 48.
- Fernandes Q, Allouch S, Gupta I, Elmakaty I, Elzawawi KE, Amarah A, et al. Human papillomaviruses-related cancers: an update on the presence and prevention strategies in the Middle East and North African regions. Pathogens, 2022; 11(11): 1380.
- Cherif S, Amine A, Thies S, Taube ET, Braicu EI, Sehouli J, et al. Prevalence of human papillomavirus detection in ovarian cancer: A meta-analysis. European Journal of Clinical Microbiology & Infectious Diseases, 2021; 40(9): 1791-1802.
- Naupu PN, van Zyl AR, Rybicki EP, Hitzeroth II. Immunogenicity of plant-produced human papillomavirus (HPV) virus-like particles (VLPs). Vaccines, 2020; 8(4): 740.
- Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines, 2020; 8(3): 391.
- Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Frontiers in Cellular and Infection Microbiology, 2022; 12: 909223.
- Paz-Zulueta M, Álvarez-Paredes L, Rodríguez Díaz JC, Parás-Bravo P, Andrada Becerra ME, Rodríguez Ingelmo JM, et al. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC cancer, 2018; 18: 1-9.
- Kargar F, Rahmati M, Jamalidoust M, Mortazavi M, Savardashtaki A, Milani M, et al. Human Papillomavirus (HPV16 and HPV18) Infection; Pathogenesis, Vaccination, and Detection by Loop-Mediated Isothermal Amplification with Lateral Flow Dipstick Tests: A Review. Archives of Pediatric Infectious Diseases, 2024 (In Press).
- Huber B, Schellenbacher C, Shafti-Keramat S, Jindra C, Christensen N, Kirnbauer R. Chimeric L2-based virus-like particle (VLP) vaccines targeting cutaneous human papillomaviruses (HPV). PLoS One, 2017; 12(1): e0169533.
- Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug safety, 2018; 41: 329-346.
- Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, et al. 4-valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics, 2017; 140(6).
- Mahmoud NA, Nofal Role of Different Immunotherapy Agents in Anogenital Warts Management. The Egyptian Journal of Hospital Medicine, 2022; 89(1): 5698-5701.
- Ahn HC, Kim DH, Cho CH, Byun JC, Cho JH. Neuralgic amyotrophy with concomitant hereditary neuropathy with liability to pressure palsy as a cause of dropped shoulder in a child after human papillomavirus vaccination: a case report. Children, 2022; 9(4): 528.
- Gari Human Papilloma Virus, its Vaccine Significance, Side Effects, and Complications. Pharmacophore, 2021; 12(4-2021): 36-40.
- Schwarz TF, Huang LM, Valencia A, Panzer F, Chiu CH, Decreux A, et al. A ten-year study of immunogenicity and safety of the AS04-HPV-16/18 vaccine in adolescent girls aged 10-14 years. Human vaccines & immunotherapeutics, 2019; 15(7-8): 1970-1979.
- Shi LW, Li J, Yu BW, Huang LR, Li K, Ji M, et al. Safety and immunogenicity of a bivalent HPV16/18 vaccine in Chinese females. Human Vaccines & Immunotherapeutics, 2023; 19(1): 2209001.
- Sette A, Crotty Immunological memory to SARS‐CoV‐2 infection and COVID‐19 vaccines. Immunological reviews, 2022; 310(1): 27-46.
- Stanley M, Joura E, Yen GP, Kothari S, Luxembourg A, Saah A, et al. Systematic literature review of neutralizing antibody immune responses to non-vaccine targeted high-risk HPV types induced by the bivalent and the quadrivalent vaccines. Vaccine, 2021; 39(16): 2214-2223.
- Prabhu PR, Carter JJ, Galloway DA. B cell responses upon human papillomavirus (HPV) infection and vaccination. Vaccines, 2022; 10(6): 837.
- Inoue T, Kurosaki T. Memory B cells. Nature Reviews Immunology, 2024; 24(1): 5-17.
- Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily MC, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet, 2019; 394(10197): 497-509.
- Hoes J, Pasmans H, Schurink-van’t Klooster TM, van der Klis FR, Donken R, Berkhof J, et al. Review of long-term immunogenicity following HPV vaccination: Gaps in current knowledge. Human vaccines & immunotherapeutics, 2022; 18(1): 1908059.

