Global Burden of Osteoarthritis and Vitamin C Research Trends, Disease Consequences, and Implications
Ray Marks*
Department of Research, Osteoarthritis Research Center, Canada
Received Date: 01/07/2024; Published Date: 10/10/2024
*Corresponding author: Dr. Ray Marks, OARC Clinical Research and Education Director, Box 5B, Thornhill, Ontario L3T 5H3, Canada
Abstract
Osteoarthritis, the most prevalent musculoskeletal disease remains relatively impervious to a desired reversal of its progressive impact on mobility and life quality. Moreover, even if available, standard treatments may not be indicated, or efficacious, or safe and commonly fail to impact the disease directly. A disease with multiple progressively degenerating physical manifestations including joint tissue damage, inflammation, and muscle pathology, antioxidants such as vitamin C may prove helpful, especially in protecting vulnerable older adults from undue pain and suffering and excess joint destruction. Building on a prior in-depth overview capturing almost all data on this topic prior to 2019, here we present some newer data on this topic published since 2019 that furthers the view that vitamin C has noteworthy anabolic and catabolic properties that can be harnessed to mediate, moderate, or even prevent osteoarthritis joint damage in later life. Its vast protective and interactive attributes with joint and brain tissues show sufficient potential in the context of osteoarthritis pain and inflammation, as well as joint structure and cartilage physiology that is of paramount importance to validate and should be intently explored to prevent overlooking a promising non narcotic osteoarthritis pain and free radical reduction source.
Keywords: Aging; Ascorbic Acid; Cartilage; Intervention; Joint Inflammation; Osteoarthritis; Pain; Vitamin C
Introduction
Osteoarthritis a widespread highly disabling joint disease affecting many older adults no matter where they reside has been and remains an immensely costly public health challenge with few means of its mitigation using safe cost-effective approaches [1]. A commonly progressive disease that affects multiple joint tissues and their components as well as their physiology especially that of the cartilage tissue protective lining joints such as the knee, osteoarthritis is a chronic disease and one strongly implicating a high presence of ROS (reactive oxygen species) known to degrade cell membranes, tissue biology and structure as well as mediate or moderate other adverse pathophysiological disease mechanisms. As a result, as the disease progresses, the affected individual commonly experiences substantive bouts of overall dysfunction, pain, joint inflammation, muscle mass declines, joint stiffness, and multiple functional limitations with immense life negating implications for the sufferer, who is often an older adult with multiple health concerns, as well as their families, health providers, health economists, and health policy makers.
In this regard, as the ability to cure osteoarthritis or reverse this debilitating condition is not possible currently in 2024, nontoxic naturally occurring intervention approaches to offset the challenges that do prevail or are anticipated are being sought quite intently. Since many pharmacologic approaches that are mainstream are also found unsafe, and most are not risk free, the body of data generated to date in this regard has revealed vitamin C, also termed ascorbic acid, and an essential highly potent water-soluble vitamin and micronutrient may hold some promise as an adjunctive strategy for enhancing the management of its symptoms [2-4].
Studied widely for its promise in this regard are indications of its potential influence in enhancing cartilage regeneration processes, while allaying fatigue [5-8]. Other potentially noteworthy actions are its ability to modulate multiple neural functions that might reduce neuropathic pain, plus its crucial role in collagen synthesis, stress control and immune functions, and health, in general [5-8].
Applied in the realm of osteoarthritis more specifically [9], and as discussed for several decades, vitamin C appears of high relevance to much needed efforts to minimize the magnitude and intensity of any prevailing joint destruction and pain that emerges over time. Moreover, the fact that oxidative damage is a potential remediable osteoarthritis moderator, speaks to why vitamin C, known to have specific antioxidant properties, can potentially counter the production of damaging pain provoking free radicals that would otherwise have multiple negative local joints as well as cognitive implications for the sufferer [8]. Alternately, its optimal presence and potentially favorable wound healing properties and others may help prevent associated or excess cartilage tissue damage, while fostering vitally important collagen synthesis processes, as well as mental health status [8].
In the face of few safe cost effective options and escalating aging populations who may suffer from osteoarthritis, this array of possibility benefits attributed to the presence of adequate vitamin C levels seems especially important to explore because while several pharmacologic treatment approaches may well be temporarily helpful in the realm of osteoarthritis care and pain control, most fail to modify or reverse the disease process and its structural progression to any known degree and thus higher doses of medication may be needed over time. This latter outcome is potentially problematic, for example in the older population where some members suffering from osteoarthritis may well be unable to tolerate these osteoarthritis mainstream drugs for any long-term duration. In some cases, the drug may prove toxic to joint tissues as well as collagen production, or have fatal side effects, for example if pain relief is dependent on narcotics. In addition to the expenses patients may have to incur, some recently tested biologically oriented therapeutic approaches have failed to show promise in slowing the rate of osteoarthritis joint space narrowing. Other data reveal some anti-inflammatory drugs can indeed hasten, rather than slow the disease process, and do not always reduce pain [10]. On the other hand, a strategy that can favorably influence the structural and functional properties of the cartilage lining of an affected joint, as well as its surrounding muscles and nerve supply, and bone in a positive way, while safeguarding or helping to foster overall physical and mental health, would be expected to be highly valuable in the context of pain relief or the prevention of excess pain, the symptom of most concern to osteoarthritis patients.
Aims
This review aimed to update the extent of support for the idea that vitamin C, an established mediator of tissue biology, growth, and development with powerful antioxidant and anti-inflammatory properties may be an influential modifiable factor in the context of efforts to minimize, modulate or mediate osteoarthritis pain in the older adult. A secondary aim was to establish whether further research appears warranted in this realm given the burden of the disease and the purported role vitamin C plays in collagen synthesis and many key enzymatic essential life-affirming biological processes, as well as metabolic, genetic, molecular, and neurological processes, implicated in osteoarthritis, an idea disputed by several researchers, but not all.
Tested is the idea of whether vitamin C has a distinct bearing on directing potential cartilage healing opportunities in osteoarthritis, as well as the pain experience (Figure 1).
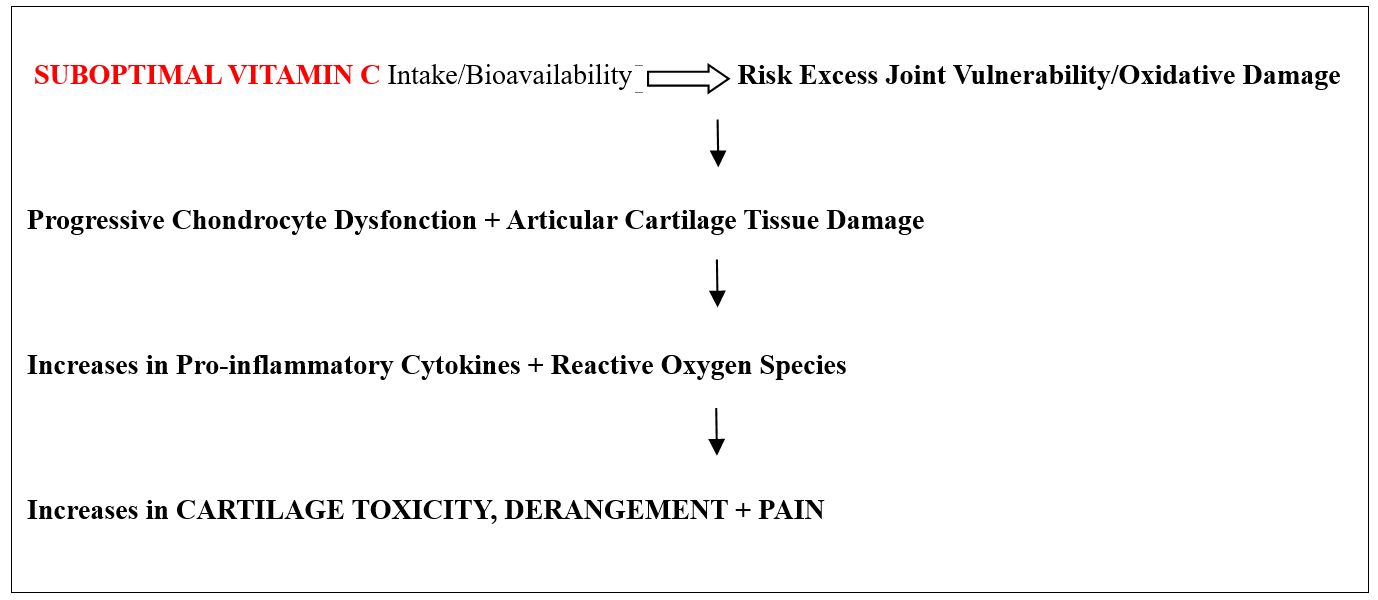
Figure 1: Hypothesis tested through this current narrative review.
Methods
To achieve these abovementioned review aims, we elected to build on an extensive review of available documents housed in PUBMED, PubMed Central, and Google Scholar from 1990 up until 2019 using the key terms Vitamin C and Osteoarthritis/Muscle/Pain. More specifically, an updated overview of related research published between January 1 2019- June 10 2024 was sought.
To this end, all available articles on these websites were scanned for relevance, and salient research articles or reviews that addressed some aspect of the current topic of interest were then reviewed in more depth without regard to research design. An attempt was made to include all modes of experimentation, but the focus was on clinically derived data as vitamin C is synthesized by almost all animals used in preclinical osteoarthritis studies so the two systems are not strictly compatible. No systematic review was conducted, and while it is acknowledged the body of data may not be exhaustive-it does arguably highlight some telling lines of research and tentative conclusions and only uses three data bases, these were taken to presumably house and capture the state of the art and gold standard papers on this topic sufficient for arriving at a reasoned opinion through a narrative lens. Readers can learn more by exploring the current cited reports as well as some prior reports [eg., Ballez et al. [11], Grover et al. [12], Choi et al. [13], and Marks [14].
Results
As of June 1, 2024, the data bases examined revealed only a small number of relevant studies, for example, 4 in PUBMED 2024 and 2020, 12 in 2021, 10 in 2022, 7 in 2023. These data are clearly very limited in number and scope as far as what is needed to address prior gaps in this realm and arrive at firm conclusions, and especially when compared to other overriding themes dealing with osteoarthritis as well as vitamin C research over the same time periods. These data also render their take home messages very challenging to unify because the reports available stem largely from cross-sectional clinical research reports, review articles, or laboratory-based studies on isolated tissues in animal models that may not replicate human osteoarthritis in the older person.
There is also clearly no past or emergent unifying underlying conceptual or theory base or consistent theme or terminology usage that can be extracted and analyzed statistically among the papers viewed. Additionally, the key words employed did not always yield research posted on the data base, but not where anticipated. Indeed, even where modest numbers of articles were posted these were generally non uniform in multiple respects, for example, some focused on deficiency effects of vitamin C in osteoarthritis, some on its possible pain relieving or provoking supplementary effect, and some on its antioxidant or pro-oxidant effect, its destructive effects or its failure to have any meaningful effect or opposing conclusions. For example, Xu et al. [15] noted vegetable intake and a diet that contains vitamin C appeared to be helpful in averting knee osteoarthritis risk, but not at high consumption levels.
Unfortunately, even if vitamin C can yield cartilage-associated protective influences, once joint destruction has commenced, challenges arise in identifying precisely what works and why because sources and dosages or intakes of vitamin C discussed in pre clinical studies, as well as some clinical studies and their comparability remains elusive at best as discussed recently by Frediani et al [16]. Whether instruments and outcomes reported in current research reports are both reliable and valid is also hard to ascertain. For example, is a vitamin C estimate that is dependent on memory or food recall and/or whether this includes or excludes supplement usage, quality and consistency of use, along with highly heterogeneous osteoarthritis clinical or tissue samples, and other design issues not problematic in its own right? Moreover, subjective measures of pain without verification must surely render the strength of any emergent relationships between vitamin C and osteoarthritis pain tentative at best even though at least two decades have been devoted to this topic in this and other related realms.
Since the resultant data do however show that vitamin C has important antioxidant properties against inflammation, as well as serving as a co-factor in for numerous biochemical reactions involved in the synthesis and assembly of cartilage collagen, and its matrix [14,17-19], it also has an affinity to exert chondroprotective and/or cartilage tissue regenerative effects [20-22], a universal agreement in this regard would undoubtedly prove of high significance. In particular, often used or administered alongside other interventions [23,24] and/or carefully studied in non clinical animal models of osteoarthritis joint damage [eg., 22] its singular effect on inflammation and other forms of pathology appears immensely noteworthy [25] as far as fostering chondrocyte proliferation and preventing articular damage [21]. As a result, several reports do suggest therefore that more should be done to determine if low vitamin C levels are pathogenic and if so, whether the presence of adequate dietary and/or supplementary levels of vitamin C or combination thereof is beneficial to an osteoarthritis sufferer [2,6,26,27] especially if muscle that houses vitamin C has already atrophied, along with structural features such as the surrounding joint ligaments, tendons, and bone quality and local and systemic inflammation prevails unabated [28-30]. It is duly observed as well that clinically relevant associations that can be harnessed quite readily are found to exist between vitamin C levels and chronic spinal pain, a common osteoarthritis complaint [31,32], and for selected cases suffering from osteoarthritis and requiring soft tissue and bone tissue enhancements it appears vitamin C has a strong bearing on the rate of post orthopedic surgical recovery processes [33,34]. Although not well studied this favorable vitamin C associated outcome may reflect the known effects of vitamin C on bone production, soft tissue injury, pain, and fracture healing among other influential factors [33].
Indeed, Chiu et al. [35] argue that these aforementioned observations while not universally observed are worthy of exploration based on valid data showing vitamin C potentially reduces pain because its presence helps to decrease apoptotic cell death processes as well as the expression of pro-inflammatory cartilage chondrocyte derived cytokines and matrix metalloprotease degrading enzymes, even at low doses [21]. Another possible pain reducing mechanism involving vitamin C may implicate an initial reduction in the production of damaging chondrocyte enzyme activity, and thus less ROS-injury provoking chondrocyte and extracellular matrix breakdown [36]. Another is the prevention of oxidative injury as well as deterioration of associated musculoskeletal supportive and functional structures [11]. Another is improved chondrocyte viability and proliferation and possible regeneration [8,21,37].
The interesting finding that 100 percent of a group of end stage osteoarthritis sufferers undergoing knee replacement surgery had deficient vitamin C levels before surgery, and this remained true in 90 percent of cases after surgery tends to imply that the presence of an inadequate level of vitamin C intake may impact unfavorably on pain as well as inflammation resolution [28]. In contrast, adequate vitamin C plasma levels have been shown to have a favorable impact on pain [38-40], plus articular cartilage tissue viability, while possibly allowing for a decreasing analgesic need, and functional benefits. Indeed, a recent report shows drug delivery of vitamin C via bio active nano-magnetic particles in conjunction with a compound termed dexamethasone does tend to yield cartilage chondroprotective benefits that could alleviate osteoarthritis symptoms and should be duly explored [41].
Discussion
Osteoarthritis, a highly disabling largely incurable joint disease and one where any form of palliative or reparative treatment and those that safely reduce pain, would be highly prized, remains largely dependent on an array of pharmacologic and/or surgical interventions of varying degrees of efficacy and effectiveness [42, 43]. In this regard, despite considerable background research on the importance of vitamin C in minimizing oxidative stress, for example that found in osteoarthritis, very little has been forthcoming in the realm of applying vitamin C associated research towards understanding osteoarthritis pain and its possible reduction or prevention in the older adult population specifically, despite several well-founded reasons for considering this possibility.
That is, despite a reasonably strong underlying rationale for believing that vitamin C is tentatively important for purposes of ensuring optimal joint health, and that older persons with osteoarthritis may be at risk for either a reduced ability to take up vitamin C or have a greater need for this vitamin than those who are not subject to inflammatory joint changes, the possibility that suboptimal vitamin C levels are related in some way to the presence of osteoarthritis pain and its severity and extent is poorly studied, when compared to other topic areas concerning osteoarthritis pathogenesis and mitigation. Reflecting a patchwork of interesting studies but with no seemingly consistent underlying hypothesis, certain parameters that may not have been considered [2], plus widely diverse modes of inquiry and design no conclusions can be drawn with any degree of certainty. Indeed, as in the past, current data continue to be largely suggestive rather than definitive despite advances in identifying and studying cellular and molecular levels of vitamin C and cartilage cellular impacts [44] and findings of an associated injury related ability to foster muscle regeneration [45].
This seems unfortunate because some promise has been forthcoming wherein several authors have noted a potentially valuable role for considering how vitamin C might mediate or moderate the highly resistant form of pain experienced by people with osteoarthritis, and its impact on function and life quality, including related bone damage when examined in the clinical or applied realm [13, 46-50]. Moreover, multiple preclinical studies strongly support the possibility of vitamin C as an adjunct for alleviating, minimizing, ameliorating, or reversing osteoarthritis cartilage damage. Vitamin C also possesses multiple additional capacities for prevention of osteoarthritis progress, including decreases in cell death and the expression of damaging pro-inflammatory cytokines, in addition to its well documented key antioxidant actions [11].
However, despite some favorable clinically applicable findings in recent years, most current researchers are calling for more carefully designed efforts to address documented design shortcomings in promising studies and to thereby foster the ability to resolve the presently divergent viewpoints concerning its efficacy as well as safety of vitamin C, as this pertains to osteoarthritis pathology and pain amelioration, where no firm conclusions prevail as of mid 2024. As in other realms of clinical research as well as preclinical explorative efforts, prospective well controlled research designs are imperative along with insightful research questions and methods of answering these current uncertainties more objectively using well defined and stringent measures, such as plasma level vitamin C assays plus pain measured at regular intervals. Studies designed to identify how older subgroups with vitamin C deficiencies may or may not respond to supplementary forms of vitamin C, rather than failing to do this is also strongly indicated as this group will likely be vulnerable to ROS injury and a high oxidative presence. Careful attention to assessing dose concentration relationships between vitamin C supplements, with and without any dietary sources, medications that may impede vitamin C anabolic processes, and pain as well as functional correlates of various osteoarthritis cases in the clinic can also help ensure that clinically meaningful relationships that emerge are robust and can be demonstrated based on validated instrumentation and reproducible methods and statistical procedures.
In the interim, what we do know is that vitamin C is clearly an essential co-factor for fostering normal collagen synthesis, including collagen X [51], a major structural element of articular cartilage, and its surrounding tissues [52], as well as for other vital physiological chondrocyte, and bone cell functions [53]. It also appears deficient vitamin C levels are associated with pain provoking inflammation that often accompanies osteoarthritis [54]. There are also vitamin C transporter deficiencies that have the potential to markedly impact cartilage cell metabolism as well as cartilage collagen production, matrix formation and assembly, significantly and adversely that could explain osteoarthritis pain modulation challenges to some degree [55]. Vitamin C presence does however appear to be accompanied by improved pain scores, while helping subjects achieve better functional outcome than not [49]. As well, surgery for osteoarthritis or joint injuries may be optimized by carefully applied vitamin C supplements [33, 34].
It is also possible that although Joseph et al. [48] discount any vitamin C impact on osteoarthritis, its efficacy as an osteoarthritis protective factor has not been revealed uniformly and examined meticulously and comprehensively so as to rule out competing factors. Although often discussed, the role of suboptimal or noxious vitamin C supplementary doses, potentially unreliable assessments, omitted assessments, and the statistical problems of applying aggregated data from limited albeit diverse samples as recorded retrospectively or on a single occasion remains. It is possible too that more attention to the notion that implies the presence of a persistent vitamin C deficiency is a potentially debilitating health factor that may inadvertently raise the risk for osteoarthritis joint damage, while retarding its recovery potential, which is poorly studied, should be considered of high import to examine. In addition, it is acknowledged such data may exist but may have been omitted inadvertently.
However, after surveying more than 150 related papers in 2018 [14] and another 50 since then in this report, wherein most were located in the world’s leading data base of PUBMED-deemed reliable and peer reviewed- it appears safe to propose that persistent vitamin C deficits appear to have the potential to accelerate or magnify any prevailing joint pain found in most older adults suffering from osteoarthritis. It may also be that an individual struggling with painful osteoarthritis who is under stress is particularly vulnerable, and has an increasing need for long-term vitamin C supplementation to minimize health challenges that provoke ROS-injury [50], even if refuted [49].
In sum, there appears to be a valid need to generate more insight into how vitamin C may be an important osteoarthritis pathogenic correlate, and to permit more adequate translation of prevailing laboratory data showing considerable promise to the clinic. In particular, research attempts designed to differentiate the association of vitamin C levels among distinctive osteoarthritis sub-groups with and without verifiable oxidative damage as well as its possible association with disease severity and age may prove especially insightful. After that, meticulously and rigorously designed studies to rule out competing hypotheses, and to avoid undesirable cross-sectional inferences that do not take into account the fact that reported vitamin C intake on a food survey delivered retrospectively may not be the same as actual time based plasma levels are indicated. Since its effects may be both disease specific, as well as dose-dependent and take weeks or months to unfold and be influenced by gender among other factors such as age and health and disease status, more data as to the importance of these interactive factors are strongly advocated [54]. It also appears that the efficacy of tailoring doses for reducing osteoarthritis pain and moderating its development as well as its adjunctive role in mediating pain holds promise and should be carefully examined.
Concluding Remarks
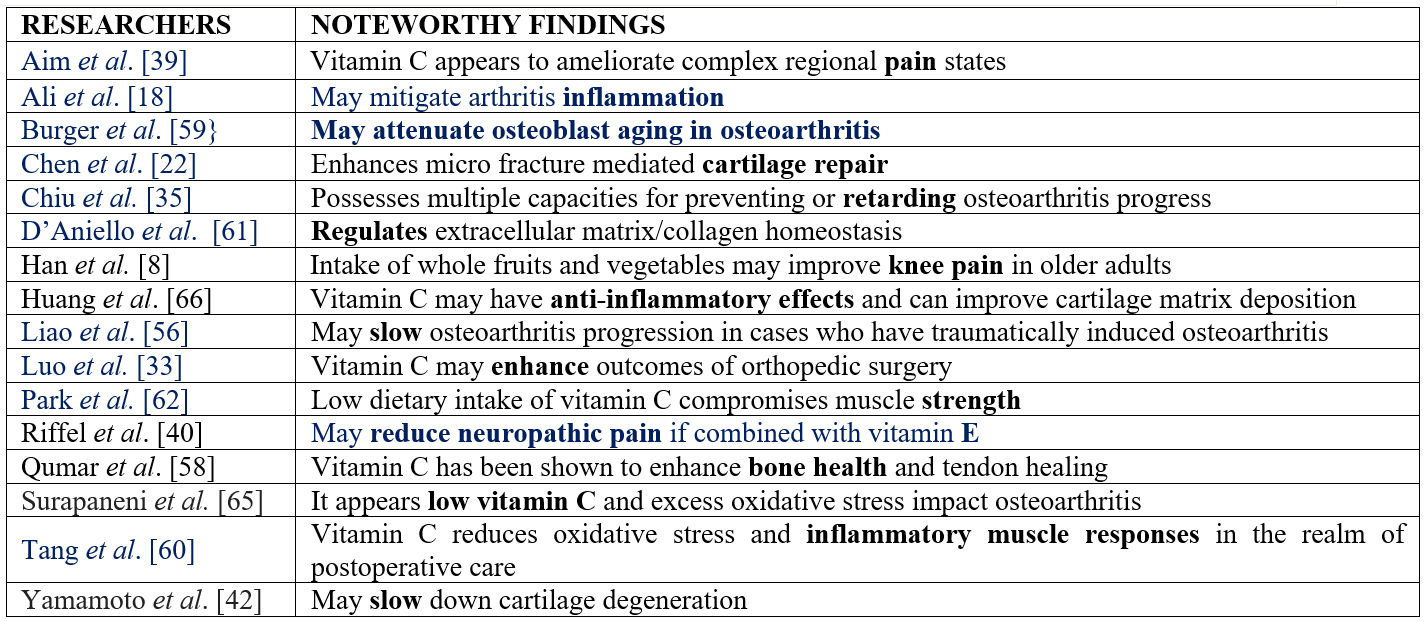
In accord with past research efforts, it seems reasonable to conclude that osteoarthritis pathology and its pain, whether at the knee joint or other joints, may be influenced in a multitude of ways by the presence or absence of optimal levels of vitamin C, even if not favorably viewed in this regard in all instances. Until more is known in this regard, and to possibly avert the degree of projected future suffering and costs associated with osteoarthritis, it appears the blood and serum vitamin C levels of older susceptible and incident cases as well as those who are disease free should be checked periodically to pinpoint any excess or borderline emergent deficit in this regard and to consider what this might predict (Table 1).
Table 1: Sample of Data Highlighting Some Possible Benefits of Attaining Optimal Daily Vitamin C Levels in Older Adults Suffering from Osteoarthritis and by Contrast Possible Adverse Impacts in the Face of Insufficient Vitamin C.
However, it is also clear that the role and possible impact of varying vitamin C levels on osteoarthritis risk, as well as osteoarthritic pain and its pathology, clearly remains to be examined and analyzed in a more substantively robust series of endeavors, including those that can detail the differential impact of various forms of delivering vitamin C and desirable dosages and their longitudinal influence on diverse joint structures, biomarkers of pain, as well as function using advanced technology. In addition, methods of assessing vitamin C should be standardized and delivery modes (listed below) should be duly studied among older adults with osteoarthritis of different degrees and vitamin C deficiencies to ascertain their independent or comparable efficacy, safety, risk and benefit profiles.
- Oral supplements of varying dosages, combinations, formulae, and usage instructions
- Intra-articular injections or delivery
- Iontophoresis using continuous electric current
- Beverage supplements
- Dietary fruit, vegetable and herbal sources
- Freeze-dried dietary powders
- Freeze-dried beverages
- Topical applications
- Laser facilitated trans dermal delivery
In short, while vitamin C clearly has the potential to play a potentially salient role in raising the risk for intractable osteoarthritis in older adults, while also having the potential to mitigate excess osteoarthritis tissue destruction and possibly repairing this, more clarification in this regard is strongly advocated. However, what is known to date is largely favorable and supports the possibly of vitamin C supplementation in the realm of mitigating some degree of osteoarthritis suffering [67-70]. Until more research is forthcoming, it appears reasonable to suggest steps should be taken by health policy makers and others were indicated to assure older adults have secure and available sources of vitamin C and are able to maintain optimal plasma vitamin C levels especially in the face of severe neuropathic type pain and excess unrelenting stress - all things considered- and even if debunked or disputed [33,48,57,60-62].
Moreover, the evidence showing vitamin C fosters multiple life affirming enzymatic processes, and decreases inflammation, while favorably influencing bone metabolism, wound healing, collagen production, and muscle strength and with measurable osteoarthritis associated pathogenic benefits, it is a possible adjunctive modes of vitamin C intervention will help those older adults with osteoarthritis to counter any deficient vitamin C levels and their potentially dire effects. This approach may also help in reducing the need for narcotics and is also likely to be more helpful than not in averting excess joint destruction that requires surgery due to its collagen building functions and chondrocyte enhancement capacity [59, 67-70]. Especially helpful in this respect is that the presence of adequate normative vitamin C levels may serve to not only foster cartilage and muscle tissue protection and with this less chances of overall functional and structural declines plus a more positive outlook rather than a negative painful and disabling array of harmful cumulative free radical oxidative insults and osteoarthritis consequences, but may help attenuate disease associated senescent processes that impact osteoarthritis bone osteoblasts adversely [59].
Benefits other than pain may include cartilage restoration and protection, bone maintenance, tendon healing, stress reduction, neural regeneration, muscle atrophy prevention, enhanced free radical scavenging, and antioxidant counter processes and their underlying mechanisms of action. Alone or in combination vitamin C presence may 1) favor overall wellbeing and reduce suffering, 2) offer a low cost widely available safe option for physically, and economically vulnerable older adults 3) reduce cases of older adult sufferers requiring placements, narcotics, or daily services, 4) foster more rapid and overall surgical recovery rates and results 5) engender fewer overall demands on health providers amidst anticipated shrinking resources and budgets, but burgeoning aging populations living to high ages and with painful disabling progressively intractable osteoarthritis.
Acknowledgements: None
Conflicts of interest: None
Funding: None
References
- Coppola Chiara, et al. Osteoarthritis: insights into diagnosis, pathophysiology, therapeutic avenues, and the potential of natural extracts. Current Issues in Molecular Biology, 2024; 46(5): 4063-4105.
- Dunlap Burton, et al. Vitamin C supplementation for the treatment of osteoarthritis: perspectives on the past, present, and future. Therapeutic Advances in Chronic Disease, 2021; 12: 20406223211047026.
- Mititelu-Tartau Liliana, et al. Editorial: vitamin C from bench to bedside. Frontiers in Nutrition, 2024; 11: 1406342.
- Takisawa Shoko, et al. Vitamin C is essential for the maintenance of skeletal muscle functions. Biology, 2022; 11(7).
- Crisol Mary, et al. Antioxidant additives reduce reactive oxygen species production in articular cartilage during exposure to cryoprotective agents. Cryobiology, 2020; 96: 114-121.
- Colletti Alessandro, Arrigo FG Cicero. Nutraceutical approach to chronic osteoarthritis: from molecular research to clinical evidence. International Journal of Molecular Sciences, 2021; 22(23): 12920.
- Dohrn Maike F, et al. Deoxy-sphingolipids, oxidative stress, and vitamin C correlate with qualitative and quantitative patterns of small fiber dysfunction and degeneration. Pain, 2022; 163(9): 1800-1811.
- Han Qian-Qian, et al. SVCT2-mediated ascorbic acid uptake buffers stress responses via DNA hydroxymethylation reprogramming of S100 calcium-binding protein A4 gene. Redox Biology, 2022; 58: 102543.
- Bharat Krish Tejas, et al. Ingredients of a natural oral nutritional supplement and their role in the treatment of osteoarthritis. Clinical Medicine Insights. Arthritis and Musculoskeletal Disorders, 2022; 15: 11795441211063365.
- Kim JR, et al. Therapeutics in osteoarthritis based on an understanding of its molecular International Journal of Molecular Science, 2018; 19(3): pii E674.
- Ballaz SJ, Rebec GV. Neurobiology of vitamin C: expanding the focus from antioxidant to endogenous neuromodulator. Pharmacology Research, 2019; 146: 104321.
- Grover AK, Samson SE. Benefits of antioxidant supplements for knee osteoarthritis: rational and reality. Nutritional Journal, 2016; 15(1).
- Choi Sooho, et al. Advances in dermatology using DNA aptamer "Aptamin C" innovation: oxidative stress prevention and effect maximization of vitamin C through antioxidation. Journal of Cosmetic Dermatology, 2020; 19(4): 970-976.
- Marks Ray. Vitamin C and osteoarthritis: mini review and commentary. CPQ Orthopaedics, 2018; 1(3): 1-16.
- Xu Chao, et al. Role of dietary patterns and factors in determining the risk of knee osteoarthritis: a meta-analysis. Modern Rheumatology, 2022; 32(4): 815-821.
- Frediani Jennifer K, et al. The role of diet and non-pharmacologic supplements in the treatment of chronic neuropathic pain: a systematic review. Pain Practice, 2024; 24(1): 186-210.
- Ramón Ricardo, et al. Anti-inflammatory effect of vitamin C during the postoperative period in patients subjected to total knee arthroplasty: a randomized controlled trial. Journal of Personalized Medicine, 2023; 13(9): 1299.
- Ali Aneesh, et al. A bioactive and biodegradable vitamin C stearate-based injectable hydrogel alleviates experimental inflammatory arthritis. Biomaterials Science, 2024; 10(10): 39/d4bm00243a.
- Schwartz ER, Adamy L. Effect of ascorbic acid on arylsulfatase activities and sulfated proteoglycan metabolism in chondrocyte cultures. Journal of Clinical Investigation, 1977; 60(1): 96-106.
- Mattiuzzo Elena, et al. In vitro effects of low doses of β-caryophyllene, ascorbic acid and d-glucosamine on human chondrocyte viability and inflammation. Pharmaceuticals, 2021; 14(3): 286.
- Shivnath Neelam, et al. Solanum xanthocarpum fruit extract promotes chondrocyte proliferation in vitro and protects cartilage damage in collagenase induced osteoarthritic rats (article reference number: JEP 114028). Journal of Ethnopharmacology, 2021; 274: 114028.
- Chen Zhian, et al. Intra-articular injection of ascorbic acid enhances microfracture-mediated cartilage repair. Scientific Reports, 2024; 14(1): 3811.
- Abpeikar Zahra, et al. Characterization of macroporous polycaprolactone/silk fibroin/gelatin/ascorbic acid composite scaffolds and in vivo results in a rabbit model for meniscus cartilage repair. Cartilage, 2021; 13(2): 1583S-1601S.
- Muftic Mirsad, et al. Evaluation of the Cartinorm use in the therapy of patients with knee osteoarthritis. Materia Socio-Medica, 2024; 36(1): 54-58.
- Shah, Shalin et al. “Vitamin C and inflammatory cytokine levels in elective total knee arthroplasty.” Nutrition and Health 26.2 (2020): 87-91.
- Delgado-Velandia, Mario et al. “Dietary vitamin C intake and changes in frequency, severity, and location of pain in older adults.” The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 93 (2024).
- Caterino, Chiara et al. Clinical efficacy of Curcuvet and Boswellic acid combined with conventional nutraceutical product: an aid to canine osteoarthritis. PloS One, 2021; 16(5): e0252279.
- Takisawa S, et al. Vitamin C deficiency causes muscle atrophy and a deterioration in physical Science Reports, 2019; 9(1): 4702.
- Valsamidou Evdokia, et al. A standardized nutraceutical supplement contributes to pain relief, improves quality of life and regulates inflammation in knee osteoarthritis patients; a randomized clinical trial. Heliyon, 2023; 9(9): e20143.
- Oakes Bennett, et al. Vitamin C in orthopedic practices: current concepts, novel ideas, and future perspectives. Journal of Orthopaedic Research, 2021; 39(4): 698-706.
- Carr AC, McCall C. The role of vitamin C in the treatment of pain: new insights. Journal of Translational Medicine, 2017; 15(1): 77.
- Dionne CE, et al. Serum vitamin C and spinal pain: a nationwide study. Pain, 2016; 157(11): 2527-2535.
- Luo Tianyi David, et al. Ascorbic acid and its clinical role in orthopaedic surgery. Journal of Surgical Orthopaedic Advances, 2018; 27(4): 261-268.
- Laumonerie P, et al. Influence of vitamin C on the incidence of CRPS-I after subacromial shoulder surgery. European Journal of Orthopaedic Surgery & Traumatology, 2020; 30(2): 221-226.
- Chiu PR, et al. Vitamin C protects chondrocytes against monosodium iodoacetate-induced osteoarthritis by multiple pathways. International Journal of Molecular Science, 2016; 18(1): pii E38.
- Zhou Meng, et al. ROS-induced imbalance of the miR-34a-5p/SIRT1/p53 axis triggers chronic chondrocyte injury and inflammation. Heliyon, 2024; 10(11): e31654.
- Zheng Kaiwen, et al. Enhanced articular cartilage regeneration using costal chondrocyte-derived scaffold-free tissue engineered constructs with ascorbic acid treatment. Journal of Orthopaedic Translation, 2024; 45: 140-154.
- Barker Tyler, et al. Multi-vitamin supplementation blunts the circulating IL-6/IL-10 ratio increase after knee arthroplasty: a randomized, double-blind, placebo controlled study. Cytokine, 2021; 140: 155435.
- Aïm F, et al. Efficacy of vitamin C in preventing complex regional pain syndrome after wrist fracture: a systematic review and meta-analysis. Orthopedic Traumatology Surgery and Research, 2017; 103(3): 465-470.
- Riffel APK, et al. Treatment with ascorbic acid and α-tocopherol modulates oxidative-stress markers in the spinal cord of rats with neuropathic pain. Brazilian Journal of Medicine and Biology Research, 2018; 1(51): e7097.
- Zară-Dănceanu, Camelia Mihaela, et al. Magnetic nanoemulsions for the intra-articular delivery of ascorbic acid and dexamethasone. International Journal of Molecular Sciences, 2023; 24(15): 11916.
- Yamamoto Nobuyuki, et al. Non-operative management of shoulder osteoarthritis: current concepts.” Journal of ISAKOS: Joint Disorders & Orthopaedic Sports Medicine, 2023; 8(5): 289-295.
- Apostu Dragos, et al. Systemic drugs with impact on osteoarthritis. Drug Metabolism Reviews, 2019; 51(4): 498-523.
- Amr Mahmoud, et al. In vitroeffects of nutraceutical treatment on human osteoarthritic chondrocytes of females of different age and weight groups. Journal of Nutritional Science, 2021; 10:
- Zhang Xiaoyu, et al. Vitamin C regulates skeletal muscle post-injury regeneration by promoting myoblast proliferation through its direct interaction with the Pax7 protein. Food & Function, 2024; 15(8): 4575-4585.
- Skalny Anatoly V, et al. Role of vitamins beyond vitamin D3in bone health and osteoporosis (Review). International Journal of Molecular Medicine, 2024; 53(1): 9.
- Uehara Hirohisa, et al. The effect of vitamin C and N-Acetylcysteine on tendon-to-bone healing in a rodent model of rotator cuff repair. The American Journal of Sports Medicine, 2023; 51(6): 1596-1607.
- Joseph Gabby B, et al. Associations between vitamins C and D intake and cartilage composition and knee joint morphology over 4 years: data from the Osteoarthritis Initiative. Arthritis Care & Research, 2020; 72(9): 1239-1247.
- Ranjbari Fatemeh, Ehsan Alimohammadi. Unveiling the potential impact of vitamin C in postoperative spinal pain. Chinese Neurosurgical Journal, 2024; 10(1): 16.
- Iliuță Daniel, et al. Research on the influence of MUVON PLUS treatment upon the biomechanical behavior of the human osteoarthritic knee. Current Health Sciences Journal, 2023; 49(1): 75-84.
- Leboy PS, et al. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. Journal of Biological Chemistry, 1989; 264(29): 17281-17286.
- Röhr D, et al. Sodium-dependent vitamin C transporter 2 deficiency impairs myelination and remyelination after injury: roles of collagen and demethylation. Glia, 2017; 65(7): 1186-1200.
- Lindsey Richard C, et al. Vitamin C effects on 5-hydroxymethylcytosine and gene expression in osteoblasts and chondrocytes: potential involvement of PHD2. PloS One, 2019; 14(8): e0220653.
- Mallah Alia, et al. Sex-specific reduction in inflammation of osteoarthritic human chondrocytes and nutraceutical-dependent extracellular matrix formation. Journal of Immunology and Regenerative Medicine, 2021; 14: 100054.
- Mobasheri A, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histology and Histopathology, 2002; 17(4): 1239-1267.
- Liao Zhenting, et al. Intra-articular injection of ascorbic acid/ferric chloride relieves cartilage degradation in rats with osteoarthritis. Journal of Southern Medical University, 2018; 38(1): 62-68.
- Sophocleous A. The role of nutrition in osteoarthritis development. Nutrients, 2023; 12(15): 4336.
- Qamar Rayed, et al. Exploring ascorbic acid's role in orthopedic practices: present theories, innovative approaches, and prospects. Cureus, 2024; 16(5): e60164.
- Burger Maximilian G, et al. Ascorbic acid attenuates senescence of human osteoarthritic osteoblasts. International Journal of Molecular Sciences, 2017; 18(12): 2517.
- Tang Pan, et al. Ascorbic acid attenuates multifidus muscles injury and atrophy after posterior lumbar spine surgery by suppressing inflammation and oxidative stress in a rat model. Spine, 2018; 43(21): E1249-E1259.
- D'Aniello Cristina, et al. Vitamin C in stem cell biology: impact on extracellular matrix homeostasis and epigenetics. Stem Cells International, 2017; 2017: 8936156.
- Park Chan Yoon, Sunhye Shin. Low dietary vitamin C intake is associated with low muscle strength among elderly Korean women. Nutrition Research (New York, N.Y.), 127(92024): 75-83.
- Takisawa Shoko, et al. Vitamin C is essential for the maintenance of skeletal muscle functions. Biology, 2022; 11(7):
- Likar Rudolf, et al. The use of high-dose intravenous L-ascorbate in pain therapy: current evidence from the literature. Pain and Therapy, 2024.
- Surapaneni Krishna Mohan, Venkataramana G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian Journal of Medical Sciences, 2007; 61(1): 9-14.
- Huang Teng-Le, et al. Synergistic effect of l-ascorbic acid and hyaluronic acid on the expressions of matrix metalloproteinase-3 and -9 in human chondrocytes. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 2018; 106(5): 1809-1817.
- Chang Zhiqiang, et al. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Molecular Medicine Reports, 2015; 12(5): 7086-7092.
- Hart Adam, et al. The role of vitamin C in orthopedic trauma and bone health. American Journal of Orthopedics (Belle Mead, N.J.), 2015; 44(7): 306-311.
- Plotnikoff Ronald, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health, 2015; 15:
- Zelfand Erica. Vitamin C, pain and opioid use disorder. Integrative Medicine (Encinitas, Calif.), 2020; 19(3): 18-29.

